ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Kumar saurav1, Manas ranjan majhi2 and Vinay kumar singh2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
MgO-Al2O3 Spinel possess low mechanical property at room and high temperature . This can be improved by partial attachment of ZrO2 to the parent spinel phase. At the attachment of these ZrO2, its mechanical properties are highly increased. It was confirmed that at high temperature monoclinic form of ZrO2 was changed. The Monoclinic form of ZrO2 was changed to tetragonal or cubic form at high temperature. The general structure of MgOAl2O3 is similar to the spinel structure AB2O4 (MgAl2O4). The article reports the preparation of zirconia –spinel (composites) using sintering method and characterization of zirconia -spinel.
Keywords |
| Pressing, Poly Vinyl alcohol, Densification, Spinel. |
INTRODUCTION |
| Spinel ( MgAl2O4) is any class of mineral having a general structure are AB2O4 where A and B occupy some or all of the octahedral and tetra- hedral side, respectively.MgO-Al2O3 having a similar characteristics to the spinel (MgAl2O4) [1]. MgAl2O4 Spinel ceramic is of significant technological interest for refractory and structural applications at elevated temperature because Spinel (MgAl2O4) is a refractory material, where no liquid formation takes place with any mixture of pure magnesia and alumina at temperature below 1900 °C. The addition of ZrO2 ceramic has significantly improved the physical and mechanical properties of MgO-Al2O3.Spinel is very important material for steel ,cement and refractory industries but at high temperature its mechanical strength is very low the strength can be improved by incorporation of ZrO2 atom in parent phase[2]. |
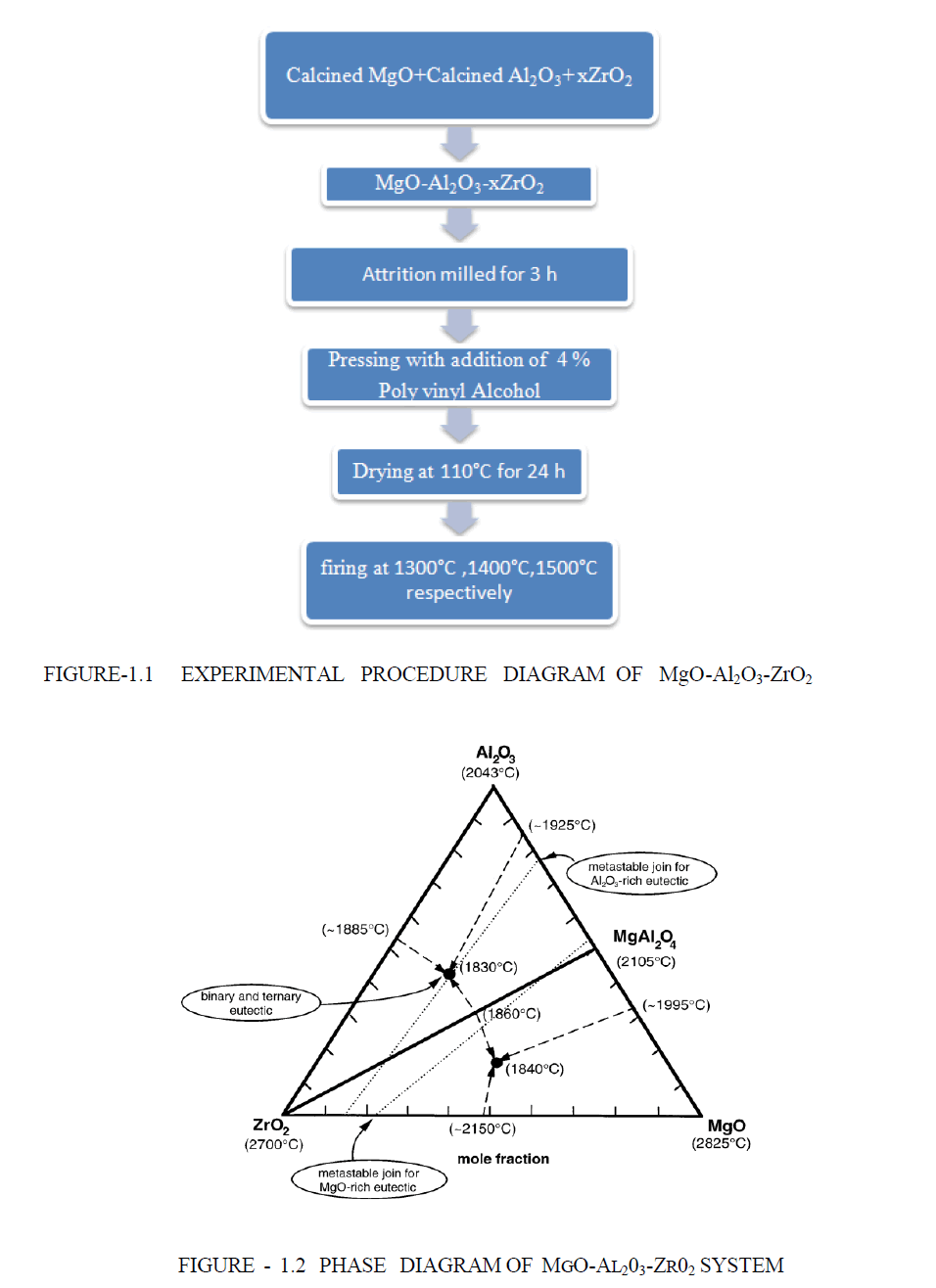
EXPERIMENTAL PROCEDURE |
| Synthesis and fabrication of Spinel MgAl2O4 is known since long. A number of techniques such as, conventional solidstate- reaction (SSR), sol-gel, spray drying (atomization) and organic gel-assisted citrate complexation, have been extensively employed . The conventional SSR method is the most utilized one in spinel preparation. |
| Lightly Calcined (fine powder) magnesia and calcined Al2O3 (fine powder) were mixed and then ZrO2 (fine powder) are mixed. The batches were comprised of MgO-Al2O3 with 1-5 contains x wt% ZrO2 where the value of x are o%, 5%, 10%, 15% 20% respectively. The batches were attrition milled for 3 h and then dried at 110°C for 24 h. The powders were shaped in pellet (piece of small shot) form using hydraulic pressing machine (uniform pressing) in the presence of 4% PVA as binder .The applied pressure are 15 tonn respectively for each sample. The samples were fired at temperatures between 1300°C and 1500°C.The heating rate was maintained at 3°C/min and soaking period was 2 hour .The Experimental procedure diagram are shown in Figure 1. The sintered sample are characterised by XRD .The Bulk density , Apparent porosity and linear shrinkage are measured the physical analysis of the sample .The addition of ZrO2 bulk density are highly increased due to the density of ZrO2 are high comparing to both Al2O3 and MgO. The apparent porosity are highly decreased. we have measured here Apparent porosity, Bulk density , linear-shrinkage ,Cold crushing strength of each composition of ZrO2 atom at temperature 1300°C,1400°C,1500°C respectively. Two ternary eutectics and one binary pseudo-eutectic (along the ZrO2– MgAl2O4 join) were identified, as shown on the Figure 1.2 [17-19]. |
 |
RESULTS AND DISCUSSION |
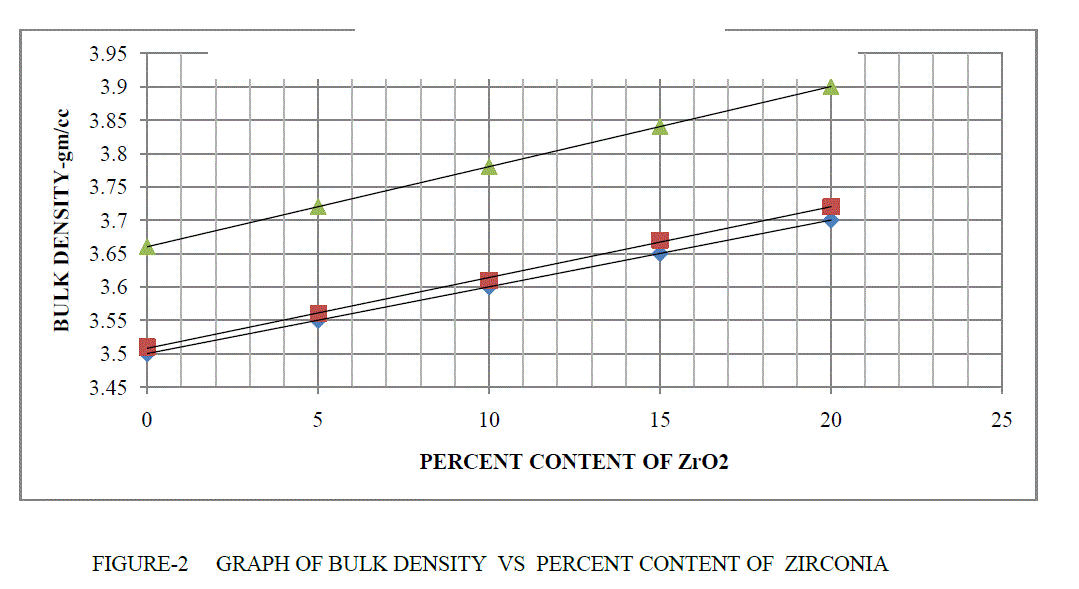
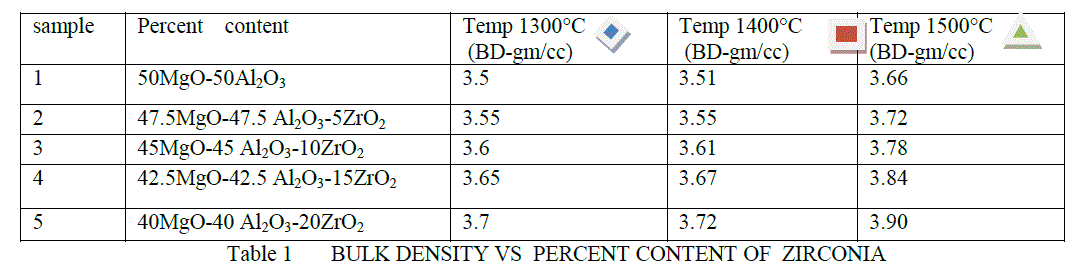
| BULK DENSITY- The bulk density of refractory materials are measured, using the Archimedes buoyancy technique with dry weights, soaked weights and immersed weights in water (mercury, xylene or denatured alcohol if the refractory is water sensitive). The plotting figure and comparing them at temperature 1300°C,1400°C,1500°C respectively at soaking 2hour. |
| BD = Dry Weight / (Soaked Weight – Suspended Weight) |
| The bulk density are shown in Table 1 and Figure 2. It is clear from above result as the percentage of ZrO2 are increased its bulk density are also increased .This is due to that because of increased densification in MgO-Al203 at high temperature . |
 |
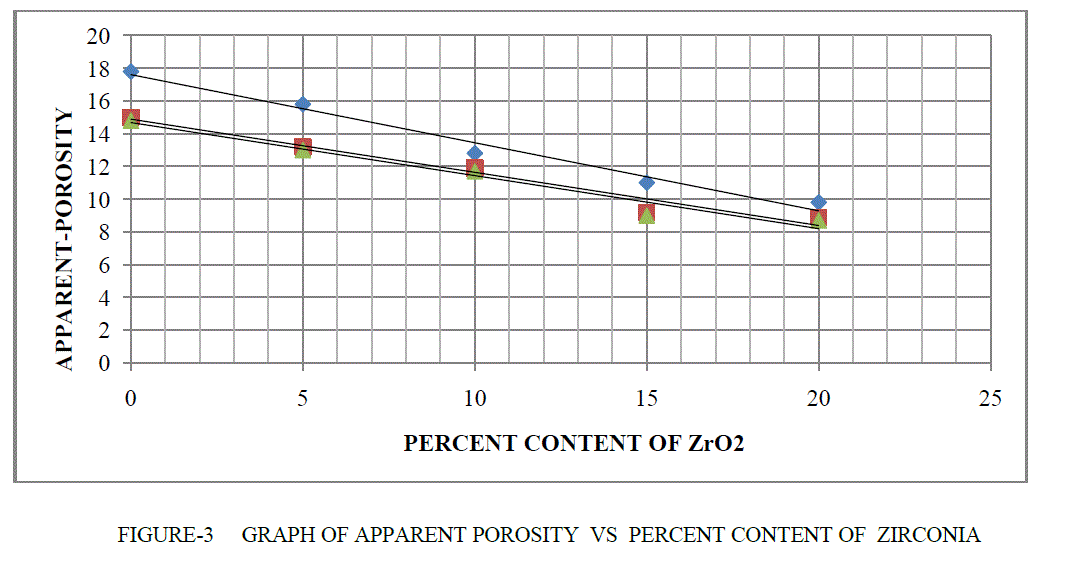
| APPARENT POROSITY- The apparent porosity of refractory material are inversely proportional of bulk density.The makeup of a porous body with solid, open pores and closed pores, and how water absorbed into the open porosity (by vacuum or boiling) presents when weighed either suspended or soaked. The Apparent Porosity, are calculated from the Dry, Soaked and Suspended weights as followsobjects, connected component labelling is applied to the resultant image.(c) represents text detection by applying second set of criteria which eliminates all the objects whose area is less than 300 and filled area is less than 500. |
CONCLUSION |
| % AP = (Soaked Weight - Dry Weight) x 100 / (Soaked Weight – Suspended Weight) |
| If percentage content of ZrO2 are increase therefore apparent porosity are decrease in MgO-Al203 -ZrO2 as shown in Table 2 and Figure 3. |
 |
MEASUREMENT OF COLD CRUSHING STRENGTH |
| The cold strength of a refractory material is an indication of its suitability for use in refractory construction. (It is not a measure of performance at elevated temperatures ) These test methods ASTM C 133 are for determining the room temperature flexural strength in 3-point bending (cold modulus of rupture) or compressive strength (cold crushing strength), or both, for all refractory products. Considerable care must be used to compare the results of different determinations of the cold crushing strength or modulus of rupture. The specimen size and shape, the nature of the specimen faces (that is, as-formed, sawed, or ground), the orientation of those faces during testing, the loading geometry, and the rate of load application, may all significantly affect the numerical results obtained. Comparisons of the results between different determinations should not be made if one or more of these parameters differ between the two determinations. The relative ratio of the largest grain size to the smallest specimen dimension may significantly affect the numerical results. For example, smaller, cut specimens containing large grains may present different results than the bricks from which they were cut. Under no circumstances should 6- by 1- by 1-in. (152- by 25- by 25-mm) specimens be prepared and tested for materials containing grains with a maximum grain dimension exceeding 0.25 in. (6.4 mm). |
| Cold crushing strength is the load at which cracks appear in the specimen. The test piece was prepared to standard size of 76.2 mm cube on a flat surface. The test piece was fired in a furnace at 1500°C, The temperature maintained for 2hours, 4hour respectively. It was cooled to room temperature and then placed on a compressive strength tester. Loads were applied axially by turning the hand wheel at a uniform rate until failure occurred. The load that caused cracks was then recorded. |
| Cold crushing strength (CCS) was calculated using the formula- |
| CCS - LOAD APPLIED / AREA |
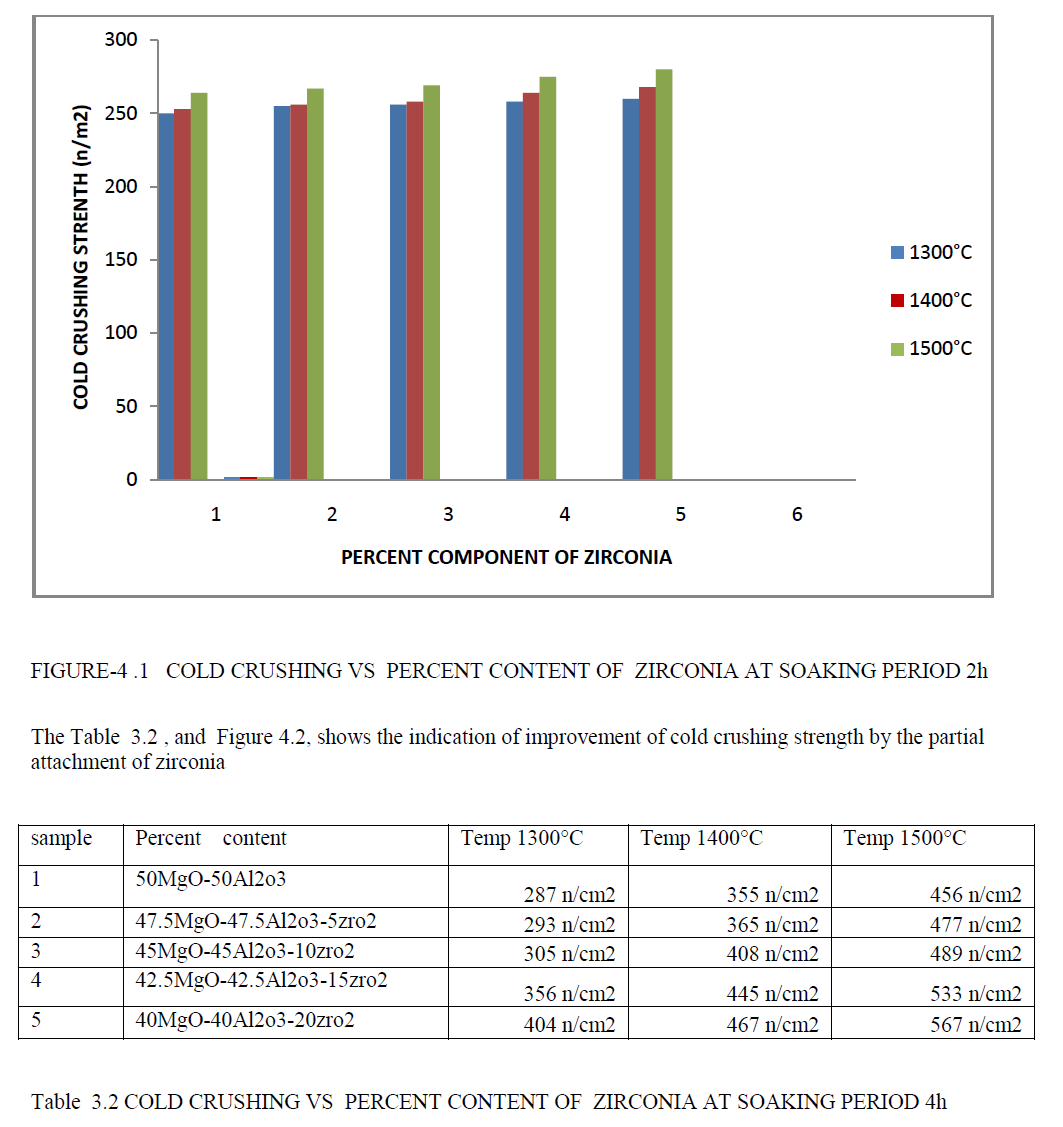
| The Table 3.1 , and Figure 4.1, shows the indication of improvement of cold crushing strength by the partial attachment of zirconia. |
 |
 |
 |
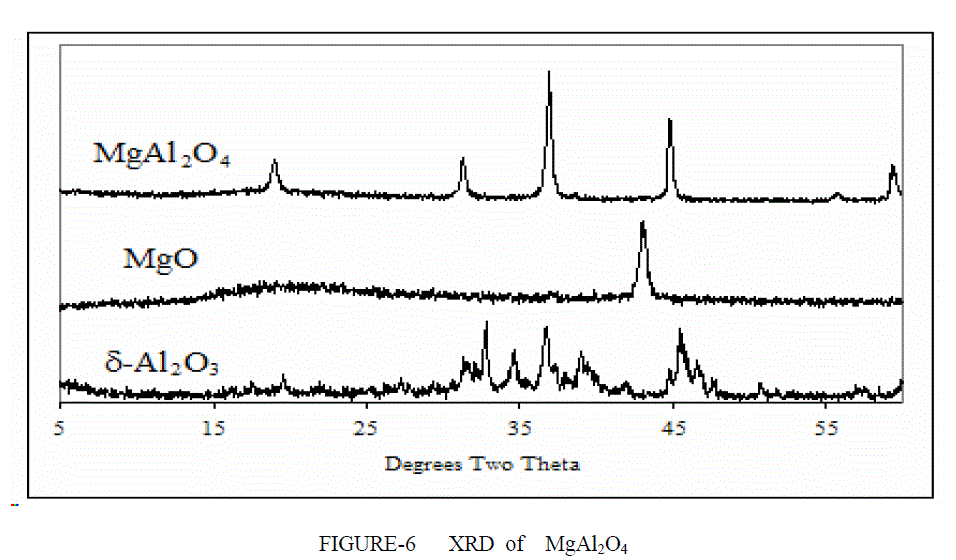 |
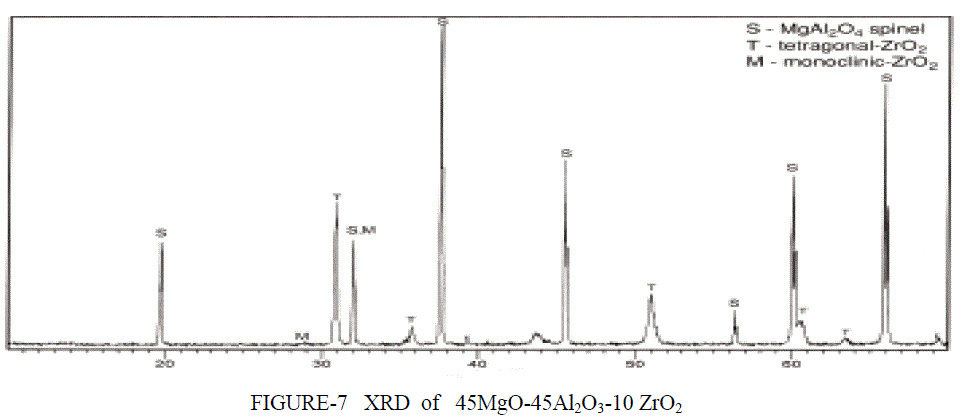
| XRD ANALYSIS- The XRD signatures of the three compositions (Al2O3, MgAl2O4, MgO) calcined at 1400°C/2 h are displayed in Figure 6. Figure 6 shows the XRD patterns of the Spinel and the support (Al2O3, MgAl2O4, MgO). The peaks at 2h = 19.1, 31.3 ,36.8, 43.0 were detected on the MgO–Al2O3 . These peaks comprise of several groups of peaks arising from different crystal phases. The peaks at 2h = 43.0 matched well to the characteristic peaks specific to MgO (PDF code: 01-079- 0612). The peaks at 2h = 19.1, 31.3, 37.0, appearing in MgAl2O4 spinel (PDF code: 01-073- 1959) and the peaks at 2h = 37.4, appearing in Al2O3 (PDF code: 00004-0880) occurred on the MgO–Al2O3 as well. Obviously, the overlapped peaks on the support broadened. This suggests that the binary MgO– Al2O3 is composed of mixed oxides of MgO, Al2O3. Theoretically, the formation of MgAl2O4 requires an equal molar ratio of MgO to Al2O3. In this study, the co-existence of Al2O3 and MgAl2O4 shows that only a part of magnesia transforms to MgAl2O4 The phase stability of the MgAl2O4 spinel has been studied by means of high-pressure X-ray diffraction for pressures up to 30 GPa. The XRD analysis of MgO-Al2O3-xZrO2 shows a different peak are obtained at different percent content of zirconia atom.. (x=10%) Comparison with the standard cards showed that all major diffraction peaks belonged to MgAl2O4.The Figure 7 shows the highest peaks are from spinel remaining peak are from tetragonal and monoclincal form of zirconia. |
 |
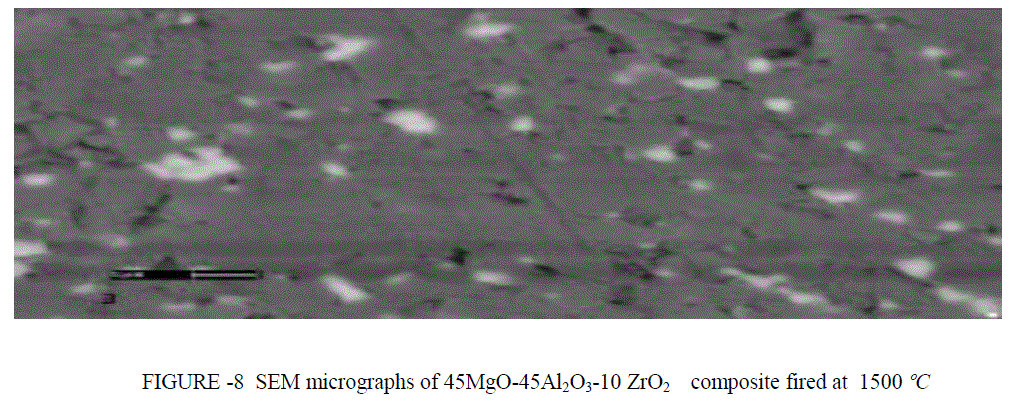
| SEM ANALYSIS- SEM micrographs of 45MgO-45Al2O3-10 ZrO2composite fired at 1400 and 1500°C were studied (Fig.8 and 9). The ZrO2 grains were small in size and occurred as intergranular grains between the MgAl2O4 grains.Grain growth of both phases was observed with increased firing temperature. Pores were eliminated at higher sintering temperature. |
 |
CONCLUSIONS |
| A high densification are classified by the partial attachment of zirconia atom at parent spinel phase It also was confirmed that densification was further improved by increased firing temperature. Increased densification with increased ZrO2 may have been caused by the presence of a discrete secondary ZrO2 phase, which retarded grain growth of the primary spinel phase. Retarded grain growth did not allow pores to become trapped within the grains. As a result, the pores did not grow in size but were eliminated from the surface of the grains. This phenomenon led to decreased porosity and improved densification of the ZrO2–MgAl2O4 composite. From the above graph |
| At different temperature, it is clear that- |
| • Where a percentage content of zirconia are increases, Bulk density are also increases. |
| • Where a percentage content of zirconia are increases Apparent porosity are decreases |
| • The percent content of zirconia are increases the Cold crushing strength of a material are increases. Improvement of Cold crushing strength due to high densification are achieved by addition of zirconia atom in parent phase spinel . |
| • The temperature are increases in zirconia- spinel ,its has a good Cold crushing strength response as comparison to normal spinel. |
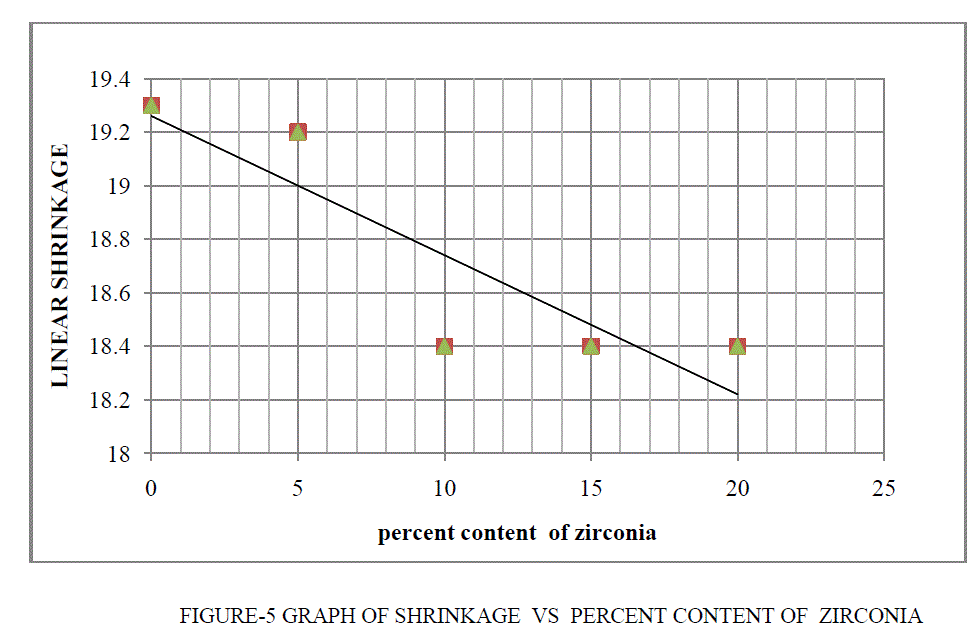
| • Percent linear shrinkage are decrease as percent content of zirconia are increases. |
References |
|