e-ISSN: 2319-9849
e-ISSN: 2319-9849
1Department of Chemistry, Government Girls PG College, Rewa, Madhya Pradesh, India
2Department of Chemistry, Government Sanjay Gandhi (Autonomous) College, Sidhi, Madhya Pradesh, India
3Department of Chemistry, Islamia College of Science and Commerce, Srinagar, Jammu and Kashmir, India
Received date: 06/09/2018 Accepted date: 09/10/2018 Published date: 11/11/2018
Visit for more related articles at Research & Reviews: Journal of Chemistry
The demi-macrocyclic ligand complex of Fe(II) ions contain N and O as donor atoms was synthesized and by FTIR and UV-Vis. Spectroscopy. The magnetic susceptibility of the complex was measured and rate was determined spectrophotometrically to verify Βeer-Lambert law at required wavelength 235 nm. The effect of pH on reaction velocity was studied. The mechanism and rate law of complex formation in consistent with the activation parameters were evaluated.
Pharmacology, Calibrants, Relaxation, Deprotonation, Vacuum
The macrocyclic and demi-macrocyclic ligands largely show vast and wide applications in various fields such as nuclear medicine, pharmacology, industry and analytical chemistry [1-4]. The natural macrocycles have been studied in considerable depth for stereochemical constrains resulting from their cyclic nature [5-7]. The metal ion is too large to fit in, to the available macrocyclic hold provided complexation occurs by either folding of metal ions or its displacement from the donor plane of the ring. It may be energetically unfavorable for their counterparts in which metal ions binds axially [8-21]. Macrocyclic ligands may also promote the formation or less coordination geometrics for particular metal ion because of increases ring strain on coordination.
Experimental
All materials in this probe were of AR-grade, BDH and Merck chemicals company. The C, H, and N elemental analysis of the sample was carried out microanalytically. Oxygen was determined by different methods, Fe(II) and chloride were determined gravimetrically. The IR spectra (4000-400 cm-1) were recorded on a Jaco model 4100 FTIR spectrophotometer as Kbr disc. whereas UV- visible spectra was recorded on shimeszu 1700. The conductivity measurements was made in DMSO or nitromethane (10-3 mho) at room temperature on Systronics conductivity meter at 8000 G in a Evans as magnetic susceptibility balance using Co[(Hg (SCN)4] as calibrant. The experimental susceptibilities were corrected for diamagnetic complex as pascal constants.
Synthesis of Ligand N2O2
Ethane 1, 2-diamine (30 g) was added to acetone (300 ml) in a 500 ml flask and the solution cooled in an ice-bath perchloric acid (72%) keeping, the temperature below 20°C. After few hours, fine crystals of the product was obtained which is insoluble in acetone, washed and vanished. The product was remained colorless and was air dried. The yield was obtained 85%.
Synthesis of Fe(II) Complex
Iron (II) perchlorate (10 gm) was dissolved in methanol followed by [amke+H2 (ClO4)] (24 gm, 0.052 mol) the mixture was refluxed. Until the color of the solution changed from green to reddish brown. After sometimes, the product was filtered recrystallized from methanol and evaporated to dryness in vacuum.
The FTIR spectra of the complex exhibits a strong sharp to medium intensity band in 500-475 cm-1 region, which may be assigned to metal-oxygen stretching vibration, the V (Fe-O) stretching frequencies. The characteristic V(C-O) absorption frequencies undergo a negative shift by about 50 cm-1 in the complex which may be assigned to the relaxation effect caused due to lone pair donation by the oxygen atom to the metal ion. The V(M-ClO4) stretching frequency. Sharp band at 535-500 cm-1 is assigned to metal nitrogen stretching (Figure 1).`
The non-appearance of Vs(N-H), Vas (N-H) complex confirm the coordination of metal ion by the deprotonation of the internal protons.
UV-vis. electronic spectra of the Fe(II) complex shows split single absorption band at 9700 cm-1 and 8690 cm-1 assignable to 5T2 → 5E transition in the ligand field of near on symmetry the D free ion ground state term splits into 5T2g ground state and 5Eg excited state. Accordingly the electronic spectra display only one spin-allowed band in the visible or near IR region. The intense CT band at 28500 cm-1 is assigned to ligand to metal C.T. (Figure 2).
The measured magnetic moment of the Fe(II) complex is in good agreement with high, spin octahedral complexes. Its value is equivalent to 5.68 B.M. The molar conductance values (λmax =5.152 cm2 mol-1) of the complex in DMSO (103 M) indicates the non-electrolyte nature.
Kinetic measurements were monitored spectrophotometrically using maximum absorption peaks at 235 nm absorption peaks at 2135 nm. A Cary model 170 dual-beam recording spectrophotometer for kinetic measurements involving both the and durum spectrophotometer at 25.0+0.2°C using circulating temperature both for all measurements, ionic strength was maintained at 0.15 M. The Beer-Lambert law [16-18] was verified from the spectral kinetic data obtained as in Table 1 (Figure 3).
| S. No. | Time (min.) | Optical density | [N2O2] × 103 (mol dm3) |
|---|---|---|---|
| 1 | 0 | 0.783 | 5 |
| 2 | 0.4 | 0.752 | 4.5 |
| 3 | 0.8 | 5.42 | 4 |
| 4 | 1.2 | 0.492 | 3.5 |
| 5 | 1.6 | 0.371 | 3 |
| 6 | 2 | 0.361 | 2.5 |
| 7 | 2.4 | 0.36 | 2.25 |
| 8 | 2.8 | 0.336 | 2 |
| 9 | 3.2 | 0.252 | 1.5 |
| 10 | 3.6 | 0.212 | 1.25 |
| 11 | 4 | 0.162 | 1 |
[Fe(II)]=5.0 × 10-2 (mol dm-3); λmax=235 nm; Temp.=298 K
Table 1. Effect of variation of concentration of N2O2 (Donor ligand) on the rate formation of demi-macrocyclic complex of Fe(II) in aqueous medium.
It is found that the increase in concentration, slightly increase the rate in the pH range from 6.55 to 9.80. The increased rate constant k1, should be much smaller than that of observed experimentally. When pH range 6.55 to 9.80 are used, the linearity of plot (Table 2). Df-Dt/time or kobs vs. [Fe(II) × 102 (mol dm-3)] or [N2O2 × 103 (mol dm-3)] for the complexation was found that, rate of reaction increases with increase in pH whereas plot of kobs versus pH gives non-linear, slightly curve type graph (Figure 4).
| S. No. | pH | [Fe L (ClO4)2] kobs × 103 (s-1) |
|---|---|---|
| 1 | 6.55 | 3.61 |
| 2 | 7 | 4.22 |
| 3 | 9.2 | 5.75 |
| 4 | 9.8 | 6.62 |
Table 2. Observed first-order rate constant kobs (s-1) for the Fe(II) demi-macrocyclic complexes with N2O2 under various pH conditions.
Complex Formation Kinetics
One kinetic process was followed:
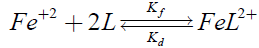 (1)
(1)
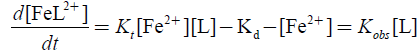 (2)
(2)
Complex formation reaction was studied under pseudo first-order conditions and 1:2 stoichiometrically, Fe(II) in excess for which rate equation may be obtained as:
Final rate law was derived as:
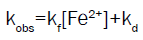 (3)
(3)
This is an excellent in conformity with the experimental kinetic facts of Fe(II)- demi-macrocycle with N2O2 complex.
Synthesis and spectral studies of demi macrocycles of donor ligand with complex of Fe (II) ion was synthesized the elemental analysis, magnetic susceptibility, FTIR and UV spectra revealed the formation of demi-macrocycles complex. Octahedral structure was assigned of Fe (II) complex ion on the basis of elemental and spectral data.