ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Tejas Babaria1, Anurag Singhania1, Stuti Khaitan1, Tejaswi1, P L Muralidhara2 and Jagadish H Patil2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Mobil crystalline material is a unique material that has propertiesof high surface area, narrow pore size distribution, high thermal stability and has wide applications in the areas of nanoscience, catalysis, environmental engineering, and adsorption and drug delivery. The present work explores to synthesizemobil crystalline material economically using free and abundantly availablerice husk as silica source and cetyltrimethyl ammonium bromide as surfactant. The product was characterized by Fourier Transform Infrared Spectroscopy (1073.91 cm-1), Nitrogen adsorption-desorption technique (2181.7 cm2/gm), pore size (27 nm) and X-ray diffraction technique (2.6oof 2θ value). The characterizations reveal the product synthesized was agood mobil crystalline material that has mesoporous molecular sieve with good adsorbing capacity.
Keywords |
| Mobil crystalline material, Cetyltrimethyl ammonium bromide, Rice husk, Nitrogen adsorption-desorption, X-ray diffraction. |
INTRODUCTION |
| Mobil Crystalline Material(MCM) is a mesoporous silicate molecular sieve which was discovered in 1992 [1],[2]. Mesoporous materials have particle size in the range of 2 nm to 50 nm. MCM is an allotrope of silicaand hasunique properties like high surface area, narrow pore size distribution, and highthermal stabilityand high hydrophobicity. MCM has been a focus for several research areas like nanoscience [3], catalysis [4], environmental engineering [5], adsorption [6] and drug delivery [7]. Yang et al. [8] introduced a rapid method for the synthesis of highly ordered mesoporous material by using silica gel as silica source. Due to high silica content in rice husk, it has become a source of preparation for a number of silicon compounds [9]. In the present work, MCM was synthesized using freely and abundantly available rice husk as a silica source and cetyltrimethyl ammonium bromide as a templating agent.The mesoporous silicate was obtained after the removal of template by calcination.Synthesis conditions such as silica source, templating agent, pH, composition of the reaction mixture and temperature determine the characteristics of the porous structure [10]-[14]. The present work focuses on effect of pHon the yield of MCM synthesis. Characterization of the porous MCM was carried out using three independent techniques namely,Fourier Transform Infrared Spectroscopy (FT-IR), nitrogen adsorption-desorption, pore size and X-Ray diffraction (XRD)[15]. |
EXPERIMENTAL METHOD |
| A. Materials |
| Cetyl Trimethyl Ammonium Bromide (CTAB), silica gel, double distilled water, ammonium hydroxide, hydrochloric acid, sodium hydroxidepurchased from Industrial and Laboratory equipment company, Bangalore India. Rice husk was procured from K R Market, Bangalore India. All the chemicals were used without further purification. |
| B. Analytical methods |
| The XRD patterns were recorded on a Philips PW 1710 automated powder diffractometer using a graphite monochromator-filtered Cu Kα radiation (R = 0.15406 nm), at a generator tension of 40 kV and generator current of 20 mA. XRD patterns for the siliceous MCMwere recorded in the range 1.5o< 2θ < 10o using a step size of 0.02o and 1 s per step, i.e., at a scan speed of 0.02o/s. |
| The BET (Brunauer, Emmett, Teller) method of calculating specific surface area from an adsorption isotherm is reliable and widely used. Surface area measurements were performed on a Micromeretics ASAP 2010 surface analyzer by adsorbing gaseous N2 at liquid nitrogen temperature. The samples (0.2-0.3 g) were degassed at 300 oC until a vacuum pressure of 2-4 μm Hg was obtained, prior to analysis. A relative pressure range (P/Po) of 0.05-0.25 was used in the analysis. |
| In FT-IR, the sample was irradiated with a spectrum of infrared radiation. Bonds have specific bond energy associated with them. The irradiated infrared rays of specific wavelengths are deflected according to the bond energy. This helps in identification of chemical bonds in the sample. Also impurities can be identified by this method. |
| C. Synthesis |
| 1) Preparation of Rice Husk Ash |
| 1 kg of rice husk was first mixed with concentrated hydrochloric acid at 1000C to leach out all the organic matter from the husk. Rice husk was then washed with water to remove excess acid and then dried for 12 hours at 1000Cin an oven. The dried rice husk was calcined at 6000C for 6 hours to obtain 92 gm of rice husk ash. |
| 2) Preparation of MCM from rice husk ash |
| 92 gm of rice husk ash was mixed with excess sodium hydroxide solution and stirred overnight to extract silica from the ash. The solution was crystallized to obtain 59.65 gm of silica. Three reaction mixtures were prepared in three 1000 ml round bottom flasks, in each flask 2.76 gm of CTAB was dissolved in 710ml of Double Distilled Water (DDW) and stirred for 20 minutes then1.68 gm of silica obtained from rice husk ash was added and stirred for 24 hours. Different amounts of ammonium hydroxide solution were added to each flask resulting in three reaction mixtures of pH8.3, 8.6 and 9. The productsobtained from each flask were filtered using a whatman filter paper and filter paper along with residues wasdried in an oven at 1000C. The dried residues in each casewere calcined at 5500C to remove the template to obtain three MCMs namely MCM 8.3, MCM 8.6 and MCM 9. The yield in each case were calculated as |
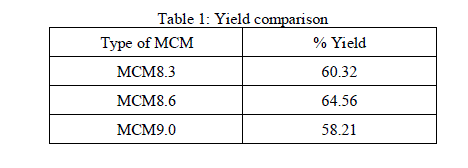 |
| It can be observed from Table 1 that the pH has significant effect on the yield of MCM. As the pH increases from 8.3 to 8.6, the yield increases, further increase of pH decreases the yield. MCM8.6 had highest yield of 64.56% and was further characterized using analytical methods. |
RESULT AND DISCUSSIONS |
| The MCM8.6 synthesized was characterized using Fourier Transform Infrared Spectroscopy (FT-IR), nitrogen adsorption-desorption, pore size and X-Ray diffraction (XRD) |
| A. FT-IR Spectroscopy |
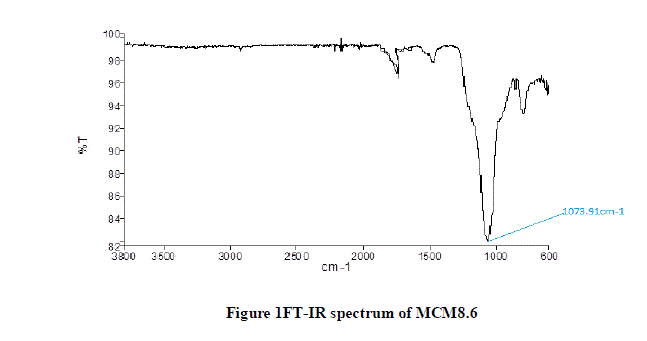
| The results of FT-IR spectroscopy for MCM8.6 are shown in Figure 1. The wavelength associated with Silica-Oxide (Si-O) bond is in the range of 1100-1000 cm-1. It can be observed from the graph the dip lies at wavelength of 1073.91cm-1. This confirms the presence of Si-O bonds in theMCM8.6. |
 |
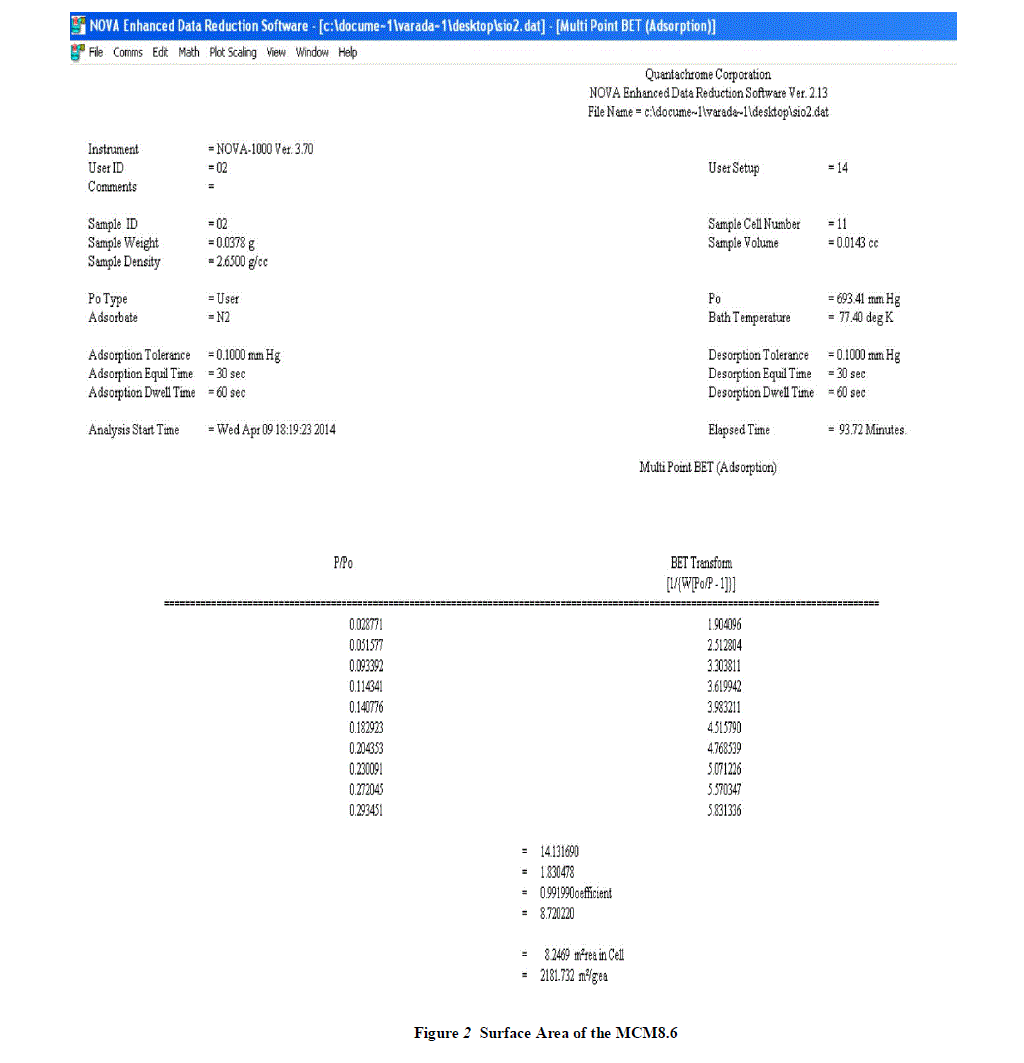 |
| C. X-Ray Diffraction Method |
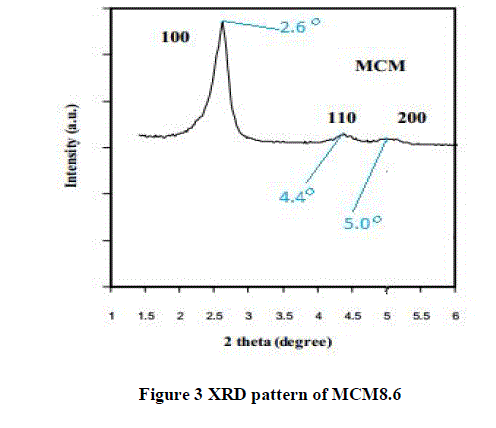
| The XRD pattern of MCM8.6is shown in Figure 3. Three reflections of silicon dioxide crystals were found at 2θ equal to 2.6°, 4.4° and 5.0° corresponding to hkl reflection planes 100, 110 and 200 respectively. These sharp signals indicated the long-range orders of the uniform hexagonal mesoporous structure. XRD patterns, shows a prominent (100) reflection peak and additional higher order peaks in the range 2o< 2θ < 7o, can provide conclusive evidence about the quality of the synthesized material. |
 |
CONCLUSION |
| Mesoporous silica materials represent a unique class of silica based materials which possess high specific surface area, large pore volume, uniform pore size (between 2-50nm), high hydrothermal stability and rich surface chemistry. MCM has been synthesized using free and abundantly available rice husk as silica source and cetyltrimethyl ammonium bromide as surfactant.at pH values 8.3, 8.6 and 9.0. The yield obtained was highest in the case of MCM 8.6. The product was characterized by Fourier Transform Infrared Spectroscopy (1073.91 cm-1), Nitrogen adsorption-desorption technique (2181.7 cm2/gm), pore size (27 nm) and X-ray diffraction technique (2.6oof 2θ value). The characterizations reveal the product synthesized was a good mobil crystalline material that has mesoporous molecular sieve with good adsorbing capacity. MCM has a diverse area of application and as a result the synthesis method needs to be modified for the mass production of it can be achieved at competitive rates. |
References |
|