ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| Nehad A. Abdel Latif 1, 2,* , Manal M. Saeed 3 , Nesreen S. Ahmed 4, 5, Rasha Z. Batran 6 and Nadia R. A. El-Mouhty 1, 7 1 Associate Professor, Chemistry Department, Faculty of Science, Taif University, 888-Taif, Kingdom Saudi Arabia 2 Associate Professor, Natural Compounds Chemistry Department, Pharmaceutical Industries Division, National Research Center, Dokki, Egypt 3 Assistant Professor, Biotechnology Deportment, Faculty of Science, Taif University, Taif, KSA 4 Assistant Professor ,Chemistry Department, Faculty of science, King Abdulaziz University, 21589jeddah, P.O. Box 80203, Saudi Arabia 5 Assistant Professor ,Medicinal Chemistry Department , National Research Center, Dokki, Cairo12622,Egypt 6 Assistant Professor, Natural Compounds Chemistry Department, Pharmaceutical Industries Division, National Research Center, Dokki, Egypt 7 Associate Professor, Labelled Compounds Department, Radioisotopes Production and Radioactive Sources Division, Hot Laboratories Center , Atomic Energy Authority, P.O. Box 13759 , Egypt |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The present research work describes the synthesis of new heterocyclic compounds using khellinone methyl ether (1) as a starting material. Compound (1) allowed to react with different aldehydes namely, benzaldehyde, p-methoxy benzaldehyde and p-nitro benzaldehyde to give the corresponding chalcones (2a-c). The latter compounds reacted with malononitrile, guanidine, ethylcyanoacetate and ethylacetoacetate to yield cyanopyridine, aminopyrimidine, cyanopyridone and cyclohexenone derivatives (3a-c), (4a-c), (5a-c) and (6a-c) respectively. When (2a-c) allowed to react with urea and thiourea, they gave oxopyrimidine and thioxopyrimidine derivatives (7a-c) and (8a-c). On the other hand compound (8a-c) condensed with 3-bromopropionic acid or chloroacetic acid to yield thiazinopyrimidine (9a-c) and 3- thiazolo-pyrimidine (10a-c) respectively. Compounds (8a-c) were condensed with chloroacetic acid and aromatic aldehyde to yield the aryl methylene derivatives (11a-c) which could be prepared directly by condensation of compound (10a-c) with aromatic aldehyde. The characterization of the resulting products were confirmed by FTIR, 1HNMR, MS and elemental analyses. The newly synthesized compounds were screened for their antibacterial activity against Escherichia coli, Pseudomonas aurignosa, Salmonella typhimurium, Bacillus subtilis and Staphylococcus aureus using the disc diffusion method.
Keywords |
| antibacterial agents, pyridine derivatives, pyrimidine derivatives, cyclohexenone derivatives, thiazinopyrimidine derivatives, and thiazolo-pyrimidine derivatives. |
INTRODUCTION |
| Chalcones, one of the major classes of natural products with widespread occurrence in fruits, vegetables, spices and soy based foodstuff, have been reported to possess several biological activities such as anti-inflammatory(1), antibacterial (2,3), anti-fungal(4-6), and anti-tumor activities(7-10). An important feature of chalcones is their ability to act as an intermediate for the synthesis of biologically active heterocyclic compounds such as, pyridine, pyrimidine, cyclohexenone, pyrazoline and isoxazoline derivatives (11,12) . pyrimidine and related pyrimidines are classes of fused heterocycles that are of considerable interest because of the diverse range of their biological properties. These are among a wide variety of nitrogen heterocycles that have been explored for developing pharmaceutically important molecules. Thiazolopyrimidine and related fused heterocycles are of interest as potential bioactive molecules, which can be considered as thia-analogues of the natural purine bases such as adenine and guanine, and have acquired a growing importance in the field of medicinal chemistry because of their biological potential. They are known to exhibit pharmacological activities such as analgesic, antiinflammatory, antiarrhythmic, antiparkinsonian, and anticancer activities (13-20). In the last several decades, fused pyrimidine derivatives are a class of heterocyclic compounds that have attracted significant interest in medicinal chemistry because they have a wide range of pharmaceutical and pharmacological applications including potential anti-tumor, antimycobacterial, and antiviral activities. Moreover, in recent years, it was reported that many fused pyrimidine analogues were reported to be inhibitors of tyrosine kinase and cyclin-dependent kinases, which are involved in mediating the transmission of mitogenic signals and numerous other cellular events (21- 26), including, cell proliferation, migration, differentiation, metabolism, and immune responses. It was also found that many of these derivatives may block proliferation of various cancer cell lines (27). Based on the above observation and in continuation of our search work (11-14) , herein we reported the synthesis of some new pyridine, pyrimidine, fused pyrimidine and cyclohexenone derivatives and evaluating their antibacterial activity. |
II- ESULTS AND DISCUSION |
| II.1. CHEMISTRY |
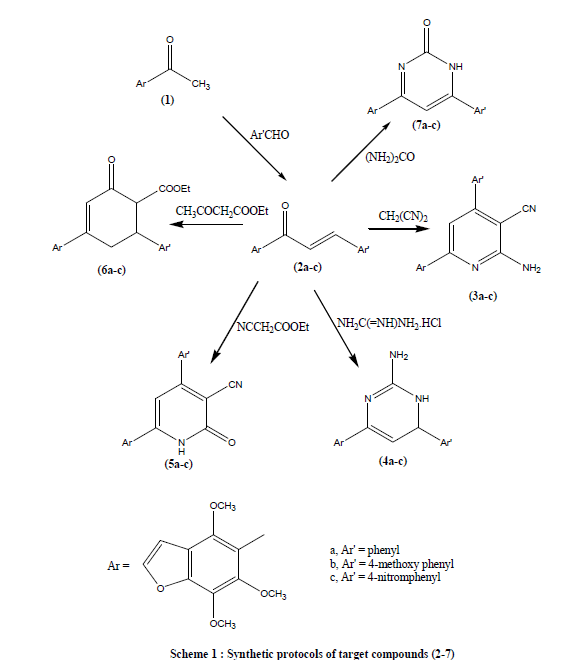
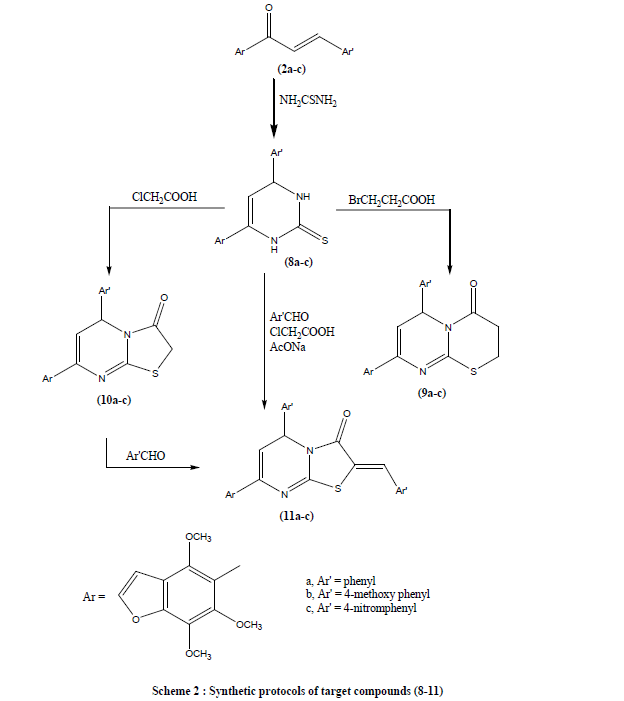
| The reaction routes for the synthesis of the title compounds were described, as shown in Schemes 1 and 2. Claisen– Schmidt reaction of khellinone methyl ether (1) with some aromatic aldehydes namely, benzaldehyde, p-methoxy or pnitrobezaldehydes in ethanol in the presence of aqueous potassium hydroxide (25%) led to the formation of 4,6,7-trimethoxy-5-( 3- substituted cinammoyl) benzofuran (2a-c) (Scheme 1). Compounds (2a-c) were confirmed by their correct elemental analyses and spectral data. Cyclocondensation of chalcones (2a-c) with malononitrile in absolute ethanol and in the presence of ammonium acetate as a catalyst to give 2-amino-3- cyano- 4-(substituted phenyl)-6- (4,6,7- trimethoxy benzofuran-5- yl) pyridine (3a-c). Reaction of chalcones (2a-c) with guanidine hydrochloride in dry ethanol in the presence of anhydrous sodium acetate yielded 2-amino- - 4-(substituted phenyl) -6- (4,6,7- trimethoxy benzofuran-5- yl) -3,4-dihydropyrimidine (4a-c) (Scheme 1). It was reported that, reaction of chalcone with ethylcyanoacetate in absolute ethanol and in the presence of ammonium acetate as a catalyst to give 2-oxo-3- cyano- 4-(substituted phenyl)- 6- (4,6,7- trimethoxy benzofuran-5- yl) -1,2-dihydropyridine (5a-c). Moreover, reaction of chalcone with ethyl acetoacetate in the presence of aqueous potassium hydroxide gives rise to ethyl-4-(4,6,7- trimethoxy benzofuran-5- yl)- 6-(substituted phenyl) -2-oxo-cyclohexa-3-ene-1-ethyl carboxylate (6a-c). Reaction of compounds (2a-c) with urea in dry ethanol in the presence of glacial acetic acid as a catalyst gave 4-(4,6,7- trimethoxy benzofuran-5- yl)-6-(substituted phenyl)-pyrimidin-2(1H)-one (7a-c) (Scheme 1). But when compounds (2a-c) reacted with thiourea yielded 4-- (substituted phenyl) -6- (4,6,7- trimethoxy benzofuran-5- yl) -1,2,3,4-tetrahydropyrimidin-2-thione (8a-c) (Scheme 2). Reaction of compounds (8a-c) bromopropionic acid and chloroacetic acid in acetic acid and acetic anhydride in the presence of anhydrous sodium acetate gave 5-(6-(substituted phenyl)-2,3-dihydro-6H-thiazino[3,2-a]-pyrimidine-4- one-8-yl)-4,6,7-trimethoxy benzofuran (9a-c) and 5-(5-(substituted phenyl)-2,3-dihydro-6H-thiazolo[3,2-a]-pyrimidin- 3-one-7-yl)-4,6,7-trimethoxy benzofuran (10a-c) respectively. Condensation of (10a-c) with aromatic aldehydes namely, benzaldehyde, p-methoxy or p-nitrobezaldehydes in acetic acid and acetic anhydride in the presence of anhydrous sodium acetate yielded 5-(2-arylmethylene-5-(substituted phenyl)-2,3-dihydro-5H-thiazolo[3,2-a]- pyrimidin-3- one-7-yl)-4,6,7-trimethoxy benzofuran (11a-c). however, compounds (11a-c) were prepared directly from (8a-c) by its reaction with chloroacetic acid, aromatic aldehydes in acetic acid and acetic anhydride in the presence of anhydrous sodium acetate (scheme 2) . |
 |
 |
| The structures of the newly synthesized compounds were confirmed on the bases of elemental analyses as well as spectral data (IR, 1HNMR and MS spectra) (c.f. Experimental section). |
| II.2. ANTIBACTERIAL ACTIVITY |
| The antibacterial activity of synthesized compounds against, Escherichia coli, Pseudomonas aurignosa, Salmonella typhimurium, Bacillus subtilis and Staphylococcus aureus were measured by measuring the zone of inhibition in disc diffusion method. the inhibition zones of microbial growth surrounding the filter paper disc (5mm) were measured in millimeters to the corresponding synthesized compounds are shown in (Table 1). The Zone of inhibition ranged from 9-30 mm. Test results are shown in (Table 1). From the data, it is clear that compounds (4b),( 6a), (7b), (9b), (10a) and (11b) possess high activity, while compounds (1), ( 2c ), ( 3c ), (4a ), (4c ), (5b ), (5c ), (6b ),(7c )(8a) , (8b) and (9a) possess moderate activity and the rest of the prepared compounds possess low activity against gram-positive strains as far as gram-negative microorganisms are concerned. |
| In our study, a wide range of microorganisms were examined, including Gram positive and Gram-negative bacteria, This may partly indicate that synthesized compounds have broad inhibitory activities to pathogenic microorganisms and are promising to act as potential antibacterial agents sources according to (Gücin et al.1997)(28). |
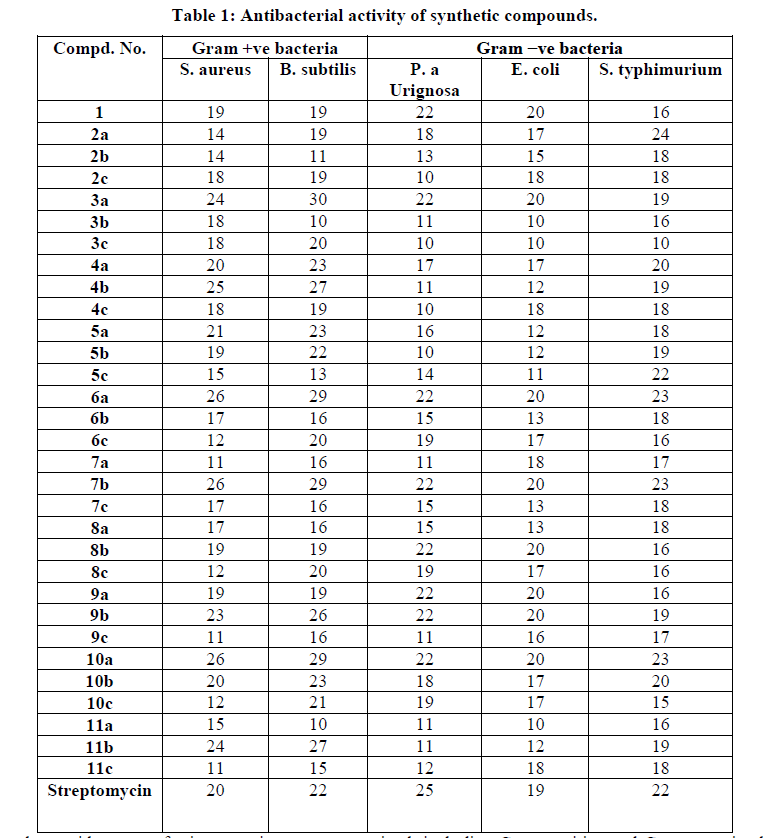 |
| In our study, a wide range of microorganisms were examined, including Gram-positive and Gram-negative bacteria, This may partly indicate that synthesized compounds have broad inhibitory activities to pathogenic microorganisms and are promising to act as potential antibacterial agents sources. |
III. ANTIBACTERIA EVALUATION |
| III.1. BACTERIAL CULTURA |
| Isolated pure culture of pathogenic bacteria, Escherichia coli, Pseudomonas aurignosa, Salmonella typhimurium, Bacillus subtilis and Staphylococcus aureus, All the strains were confirmed by cultural and biochemical characteristics and maintained in slants for further use. |
| III.2. EVALUATION OF ANTIBACTERIAL ACTIVITY |
| Antibacterial activity of each synthesized compounds was determined using a disc diffusion method(29,30). Briefly, 100 μl of the test bacteria were grown in 10 ml of fresh media until they reached a count of approximately 108 cells/ml for bacteria, 100 μl of microbial suspension was spread onto the Nutrient agar plates. The synthesized compounds were tested using 5 mm sterilized filter paper discs. Discs were impregnated with 100 μl ( 0.1 mg/mL concentration) of the test samples (acetone as Solvent), allowed to dry and placed onto inoculated plates (30 min incubation). The plates were allowed to stand at 4οC for 2 hours before incubation with the test microbial agents. Plates inoculated with Escherichia coli, Pseudomonas aurignosa, Salmonella typhimurium, Bacillus subtilis and Staphylococcus aureus were incubated at 37οC for 24 hours, than the diameters of the inhibition zones were measured in millimeters. Each antibacterial assay was performed in triplicate and mean values were reported. Standard antibiotics, streptomycin (10 served as positive controls for antibacterial activity. Filter discs impregnated with 10 μl of distilled water were used as a negative control. Solvent control disc (acetone) was also placed with the test, positive and negative control. |
IV- EXPERIMENTAL |
| IV.1.GENERAL |
| The melting points were taken in electro thermal capillary melting points apparatus and are uncorrected. Thin layer chromatography were performing using HF254 fluorescent silica gel plates (Merck) , which were examined under UV254 and 365 nm light. Silica gel (230-400 mesh) was used for flash chromatography separations. The elemental analysis for C, H, N and S were done on Vario EL III , Micro analytical center ,Cairo University, Cairo, Egypt. Infrared spectra (ν,cm-1) were recorded on Jasco FT-IR 4100 instruments using KBr Disks, Micro analytical center ,Cairo University, Cairo, Egypt. 1HNMR spectra were recorded on Varion Mercury VX-300 NMR spectrometer . 1HNMR were run at 300 MHZ in deuterated chloroform (CDCl3) or dimethyl sulphoxide (DMSO-d6). Chemical shifts are quoted in δ and were related to that of the solvents. Spectra were internally referenced to TMS. The mass spectra were recorded on Shimadzu GCMS-QP-1000 EX mass spectrometer at 70 e.V. Micro analytical center, Cairo University, Cairo, Egypt. |
| IV.2. General procedure of 4,6,7-trimethoxy-5-( 3-substituted cinammoyl) benzofuran (2a-c) A Solution of 4,6,7-trimethoxy -5-acetyl benzofuran (1 ) (0.01 mol ) in 30 ml ethanol, one gram sodium hydroxide dissolved in 3 ml water and the appropriate aromatic aldehyde ( 0.01 mol ) was stirred for one hour at room temperature and left overnight. After acidification of the reaction mixture with dilute hydrochloric acid, the formed solid was filtered off, washed with water, air dried and crystallized from ethanol. |
| 4,6,7-trimethoxy-5-( 3- cinammoyl) benzofuran (2a) m.p. 130-132 0C ; yield ( 83% ); FT-IR: ν (cm-1) 3035 (C-H aromatic), 2930 ( C-H aliphatic) and 1565 (C=C) and 1707 (C=O). 1H-NMR (δ, ppm): 7.97 ( 1H, d, H-2 furan ), 7.53 ( 5H, m, Ar-H ) , 7.03 ( 1H, d, H-3 furan ), 6.99, 6.78 (2H, 2d, CH=CH), 4.3 ( 3H, s, OCH3 ), 4.1 ( 3H, s, OCH3 ) and 3.9 ( 3H, s, OCH3 ), Mass spectra m/z (%):338 (15) [M+.], 220 (51.3), 205 (36.6), 179 (32.5), 123 ( 57.5) and 108 (100). Anal.Calcd. for C20H18O5 (338.35): C, 70.99; H, 5.36, Found: C, 70.97; H, 5.33 |
| 4,6,7-trimethoxy-5-[ 3-(p-metoxy cinammoyl)] benzofuran (2b) m.p.144 – 146 0C ; yield ( 75 % ); FT-IR: ν (cm-1) 3052 (C-H aromatic), 2965( C-H aliphatic) and 1570 (C=C) and 1726 (C=O). 1H-NMR (δ, ppm): 7.97 ( 1H, d, H-2 furan ), 7.53 ( 5H, m, Ar-H ) , 7.03 ( 1H, d, H-3 furan ), 6.97, 6.75 (2H, 2d, CH=CH), 4.3 ( 3H, s, OCH3 ), 4.1 ( 3H, s, OCH3 ), 3.9 ( 3H, s, OCH3 ) and 3.7 ( 3H, s, OCH3 ), Mass spectra m/z (%): 338 (15) [M+.], 220 (51.3), 205 (36.6), 179 (32.5), 123 ( 57.5) and 108 (100). Anal.Calcd. for C21H20O6 (368.13): C, C, 68.47; H, 5.47, Found: C, 68.45; H, 5.44 4,6,7- trimethoxy-5-[ 3-(p-nitro cinammoyl)] benzofuran (2c)m.p. 122 -1240C ; yield (69% ); FT-IR: ν (cm-1) 3066 (C-H aromatic), 2976( C-H aliphatic) and 1565 (C=C) and 1738 (C=O). 1H-NMR (δ, ppm): 7.90 ( 1H, d, H-2 furan ), 7.51 ( 5H, m, Ar-H ) , 7.19 ( 1H, d, H-3 furan ), 7.01, 6.71 (2H, 2d, CH=CH), 4.28 ( 3H, s, OCH3 ), 4.09 ( 3H, s, OCH3 ) and 3.88 ( 3H, s, OCH3 ), Mass spectra m/z (%):383 (15) [M+.], 220 (51.3), 205 (36.6), 179 (32.5), 123 ( 57.5) and 108 (100). Anal.Calcd. for C20H17NO7 (383.35): C, 62.66; H, 4.47; N, 3.65, Found: C, 62.64; H, 4.45; N, 3.63 |
| IV.3. General procedure of 2-amino-3- cyano- 4-(substituted phenyl)-6- (4,6,7- trimethoxy benzofuran-5- yl) pyridine (3a-c) A mixture of compounds (2a-c) (2mmol), malononitrile (2mmol) and ammonium acetate (0.3 g, 4mmol) in absolute ethanol (30ml) was refluxed for 3-5 hr's. after cooling, the formed product was collected by filtration, washed with ethanol, dried and crystallized from the proper solvent to give the title compounds. 2-amino-3- cyano- 4-( phenyl)-6-(4,6,7- trimethoxy benzofuran-5- yl) pyridine (3a) m.p. 197-181 0C ; yield ( 88% ); FT-IR: ν (cm-1) 3039 (C-H aromatic), 2980 ( C-H aliphatic), 2225 (cyano group), 1590 (C=C), 1666 (C=N) and 3410 (NH2). 1H-NMR (δ, ppm): 7.97 ( 1H, d, H-2 furan ), 7.53-7.41 (6H, m, Ar-H and pyridine proton) 7.03 ( 1H, d, H-3 furan ), 4.28 ( 3H, s, 2 OCH3 ), 4.19 ( 3H, s, 2 OCH3 ), 3.97 ( 3H, s, 2 OCH3 ), and 11.3 (2H, s , NH2 which is exchangeable by using D2O). Mass spectra m/z (%): 401 (27) [M+.], 325(34), 300 (64), 285 (28) and 208 (100). Anal.Calcd. for C23H19N3O4 (401.41): C, 68.82; H, 4.77; N, 10.47 Found: C, 68.79; H, 4.75; N, 10.45 |
| 2-amino-3-cyano-4-( 4-methoxyphenyl)-6-(4,6,7-trimethoxy benzofuran-5-yl) pyridine (3b) m.p. 201-203 0C ; yield ( 79 % ); FT-IR: ν (cm-1) 3059 (C-H aromatic), 2977 ( C-H aliphatic), 2228 (cyano group), 1589 (C=C), 1669 (C=N) and 3399 (NH2). 1H-NMR (δ, ppm): 7.95 ( 1H, d, H-2 furan ), 7.50-7.43 ( 5H, m, Ar-H and pyridine proton) 7.1 ( 1H, d, H-3 furan ), 4.2 ( 3H, s, OCH3 ), 4.1 ( 3H, s, OCH3 ), 3.9 ( 3H, s, OCH3 ), 3.79 ( 3H, s, OCH3 ) and 10.1 (2H, s, NH2 which is exchangeable by using D2O). Mass spectra m/z (%): 431 (23) [M+.], 406 (12), 300(100) and 208 (45). Anal.Calcd. for C24H21N3O5 (431.44): C, 66.81; H, 4.91; N, 9.74, Found: C, 66.80; H, 4.89; N, 9.70 |
| 2-amino-3- cyano- 4-( 4-nitrophenyl)-6-(4,6,7- trimethoxy benzofuran-5- yl) pyridine (3c) m.p. 195-197 0C ; yield ( 86 % ); FT-IR: ν (cm-1) 3034 (C-H aromatic), 2998 ( C-H aliphatic), 2220 (cyano group), 1577 (C=C), 1674 (C=N) and 3401 (NH2). 1H-NMR (δ, ppm): 7.99 ( 1H, d, H-2 furan ), 7.59-7.48 (5H, m, Ar-H and pyridine proton) 7.0 ( 1H, d, H-3 furan ), 4.3 ( 3H, s, 2 OCH3 ), 4.2 ( 3H, s, 2 OCH3 ), 3.96 ( 3H, s, 2 OCH3 ), and 13.3 (2H, s , NH2 which is exchangeable by using D2O). Mass spectra m/z (%): 446 (35) [M+.], 431(12), 406 (34), 285 (65) and 208(100). Anal.Calcd. for C23H18N4O6 (446.12): C, 61.88; H, 4.06; N, 12.55 Found: C, 61.85; H, 4.03; N, 12.52 |
| IV.4. General procedure of 2-amino- - 4-(substituted phenyl) -6- (4,6,7- trimethoxy benzofuran-5- yl) -3,4- dihydropyrimidine (4a-c) : |
| A solution of compounds (2a-c) (2mmol), sodium acetate (0.2 g in 1 ml water) in 30 ml ethanol, guanidine hydrochloride (0.2 g, 2mmol) was added. The reaction mixture was refluxed for 5-7 hr's. after cooling, the solid formed was collected by filtration, dried and crystallized from the proper solvent to give the title compounds. |
| 2-amino-4-phenyl-6-(4,6,7-trimethoxy benzofuran-5-yl) -3,4- dihydropyrimidine (4a) : m.p. 188-190 0C ; yield ( 91 % ); FT-IR: ν (cm-1) 3055 (C-H aromatic), 2978 ( C-H aliphatic), 1570 (C=C), 3401 and 3380 (NH2 and NH ). 1H-NMR (δ, ppm): 7.97 ( 1H, d, H-2 furan ), 7.55-7.43 (6H, m, Ar-H and Hb pyrimidine ) , 5.30(1H, d, Ha pyrimidine proton), 7.16 ( 1H, d, H-3 furan ), 4.21 ( 3H, s, OCH3 ), 4.1 ( 3H, s, OCH3 ), 3.91 ( 3H, s, OCH3 ), 13.3 (2H, s , NH2 which is exchangeable by using D2O) and 11.5 (1H, s , NH which is exchangeable by using D2O). Mass spectra m/z (%): 379 (74) [M+.], 364 (35), 288 (100) and 208 (61). Anal.Calcd. for C21H21N3O4 (379.41): C, 66.48; H, 5.58; N, 11.08, Found: C, 66.45; H, 5.55; N, 11.07 |
| 2-amino-4-(4-methoxyphenyl)-6- (4,6,7- trimethoxy benzofuran-5- yl) -3,4-dihydropyrimidine (4b) : m.p. 177-179 0C ; yield ( 68 % ); FT-IR: ν (cm-1) 3068 (C-H aromatic), 2956 ( C-H aliphatic), 1537 (C=C), 3409 and 3387 (NH2 and NH ). 1H-NMR (δ, ppm): 8.01 ( 1H, d, H-2 furan ), 7.49-7.40 (5H, m, Ar-H and Hb pyrimidine ) , 5.30(1H, d, Ha pyrimidine proton), 7.27 ( 1H, d, H-3 furan ), 4.3 ( 3H, s, OCH3 ), 4.16 ( 3H, s, OCH3 ), 3.98 ( 3H, s, OCH3 ), 3.81 ( 3H, s, OCH3 ) 12.9 (2H, s , NH2 which is exchangeable by using D2O) and 9.7 (1H, s , NH which is exchangeable by using D2O). Mass spectra m/z (%):409 (45) [M+.], 379 (45), 364 (23), 288 (16) and 208 (100). Anal.Calcd. for C22H23N3O5 (409.16): C, 64.54; H, 5.66; N, 10.26, Found: C, 64.54; H, 5.66; N, 10.26 |
| 2-amino-4-(4-nitrophenyl) -6- (4,6,7- trimethoxy benzofuran-5- yl) -3,4-dihydropyrimidine (4c) : m.p. 185-187 0C ; yield (79%); FT-IR: ν (cm-1) 3075 (C-H aromatic), 2974 ( C-H aliphatic), 1568 (C=C), 3397 and 3320 (NH2 and NH ). 1H-NMR (δ, ppm): 7.99 ( 1H, d, H-2 furan ), 7.56-7.45 (5H, m, Ar-H and Hb pyrimidine ) , 5.30(1H, d, Ha pyrimidine proton), 7.35 ( 1H, d, H-3 furan ), 4.24 ( 3H, s, OCH3 ), 4.09 ( 3H, s, OCH3 ), 3.88 ( 3H, s, OCH3 ), 13.3 (2H, s , NH2 which is exchangeable by using D2O) and 10.87 (1H, s , NH which is exchangeable by using D2O). Mass spectra m/z (%): 424 (67) [M+.], 409 (75), 364 (49) and 288 (100). Anal.Calcd. for C21H20N4O6 (424.41): C, 59.43; H, 4.75; N, 13.20, Found: C, 59.40; H, 4.72; N, 13.22 |
| IV.5. General procedure of 2-oxo-3- cyano- 4-(substituted phenyl)-6- (4,6,7- trimethoxy benzofuran-5- yl) -1,2- dihydropyridine (5a-c) |
| A mixture of compounds (2a-c) (2mmol), ethylcyanoacetate (2mmol) and ammonium acetate (0.3 g, 4mmol) in absolute ethanol (30 ml) was refluxed for 3-5 hr's. after cooling, the formed product was collected by filtration, washed with ethanol, dried and crystallized from the proper solvent to give the title compounds. |
| 2-oxo-3- cyano- 4- phenyl -6- (4,6,7- trimethoxy benzofuran-5- yl) -1,2-dihydropyridine (5a) m.p. 178-181 0C ; yield (69% ); FT-IR: ν (cm-1) 3059 (C-H aromatic), 2960 ( C-H aliphatic), 2216 (cyano group), 1579 (C=C), 1699 (C=O) and 3399 (NH). 1H-NMR (δ, ppm): 7.93 ( 1H, d, H-2 furan ), 7.48-7.37 ( 6H, m, Ar-H and pyridine proton) 7.09 ( 1H, d, H-3 furan ), 4.34 ( 3H, s, OCH3 ), 4.19 ( 3H, s, OCH3 ), 3.93 ( 3H, s, OCH3 ), and 12.3 (1H, s , NH which is exchangeable by using D2O). Mass spectra m/z (%): 402 (45) [M+.], 377 (38), 363 (49) and 287 (100). Anal.Calcd. for C23H18N2O5 (402.40): C, 68.65; H, 4.51; N, 6.96, Found: C, 68.62; H, 4.50; N, 6.93 |
| 2-oxo-3- cyano- 4-(4-methoxy phenyl)-6- (4,6,7- trimethoxy benzofuran-5- yl) -1,2-dihydropyridine (5b) m.p. 203-205 0C ; yield (73% ); FT-IR: ν (cm-1) 3065 (C-H aromatic), 2978 ( C-H aliphatic), 2220 (cyano group), 1574 (C=C), 1705 (C=O) and 3385 (NH). 1H-NMR (δ, ppm): 7.97 ( 1H, d, H-2 furan ), 7.41-7.29 ( 5H, m, Ar-H and pyridine proton) 7.11 ( 1H, d, H-3 furan ), 4.29 ( 3H, s, OCH3 ), 4.1 ( 3H, s, OCH3 ), 3.90 ( 3H, s, OCH3 ), 3.78 ( 3H, s, OCH3 ) and 12.3 (1H, s , NH which is exchangeable by using D2O). Mass spectra m/z (%): 432 (45) [M+.], 402 (35), 377 (28), 363 (59) and 287 (100). Anal.Calcd. for C24H20N2O6 (432.13): C, 66.66; H, 4.66; N, 6.48, Found: C, 66.63; H, 4.65; N, 6.45 |
| 2-oxo-3- cyano- 4-(4-nitro phenyl)-6- (4,6,7- trimethoxy benzofuran-5- yl) -1,2-dihydropyridine (5c) m.p. 198-201 0C ; yield (78% ); FT-IR: ν (cm-1) 3066 (C-H aromatic), 2989( C-H aliphatic), 2212 (cyano group), 1580 (C=C), 1701 (C=O) and 3383 (NH). 1H-NMR (δ, ppm): 7.89 ( 1H, d, H-2 furan ), 7.52-7.45 ( 5H, m, Ar-H and pyridine proton) 7.24 ( 1H, d, H-3 furan ), 4.32 ( 3H, s, OCH3 ), 4.20 ( 3H, s, OCH3 ), 3.89 ( 3H, s, OCH3 ), and 13.1 (1H, s , NH which is exchangeable by using D2O). Mass spectra m/z (%): 447 (13) [M+.], 402 (86), 377 (53), 301 (61) and 287 (100). Anal.Calcd. for C23H17N3O7 (447.40): C, 61.75; H, 3.83; N, 9.39, Found: C, 61.71; H, 3.80; N, 9.37 |
| 4.6. General procedure of ethyl-4-(4,6,7- trimethoxy benzofuran-5- yl)-6-(substituted phenyl) -2-oxo-cyclohexa- 3-ene-1-ethyl carboxylate (6a-c) A mixture of chalcones (2a-c) (0.01mol), ethylacetoacetate (0.01mol) in 10 ml absolute ethanol containing aqueous potassium hydroxide solution (1ml,10%). The reaction mixture was refluxed for 2 hr and then left overnight at room temperature. The solid that formed was collected by filtration, air dried and crystallized from absolute ethanol to give the title compounds. |
| Ethyl-4-(4,6,7- trimethoxy benzofuran-5- yl)-6- phenyl--2-oxo-cyclohexa-3-ene-1-ethyl carboxylate (6a) m.p. 198-200 0C ; yield (71% ); FT-IR: ν (cm-1) 3066 (C-H aromatic), 2985 ( C-H aliphatic), 1579 (C=C), 1699 and 1750 (C=O). 1H-NMR (δ, ppm): 7.90 ( 1H, d, H-2 furan ), 7.54 ( 5H, m, Ar-H ) , 7.12 ( 1H, d, H-3 furan ), 6.65 (1H, s, CH=C cyclohexenone), 4.31 ( 3H, s, OCH3 ), 4.15 ( 3H, s, OCH3 ), 3.90 ( 3H, s, OCH3 ), 3.5 ( 4H, m, cyclohexenone protons), 3.1 (2H, q, CH2-CO) and 2.3 (3H, t, CH3-CH2CO) . Mass spectra m/z (%): 450 (12) [M+.], 378 (68), 302 (38) and 288 (100). Anal.Calcd. for C26H26O7 (450.17): C, 69.32; H, 5.82, Found: C, 69.32; H, 5.82 |
| Ethyl -4-(4,6,7- trimethoxy benzofuran-5- yl)-6-(4-methoxy phenyl) -2-oxo -cyclohexa-3-ene-1-ethyl carboxylate (6b) m.p. 211-214 0C ; yield (69% ); FT-IR: ν (cm-1) 3059 (C-H aromatic), 2983 ( C-H aliphatic), 1580 (C=C), 1711 (C=O) and 1770 (C=O). 1H-NMR (δ, ppm): 7.93 ( 1H, d, H-2 furan ), 7.44 ( 4H, dd, Ar-H ) , 7.23 ( 1H, d, H-3 furan ), 6.62 (1H, s, CH=C cyclohexenone), 4.24 ( 3H, s, OCH3 ), 4.18 ( 3H, s, OCH3 ), 3.93 ( 3H, s, OCH3 ), 3.84 ( 3H, s, OCH3 ), 3.7 ( 4H, m, cyclohexenone protons), 3.3 (2H, q, CH2-CO) and 2.1 (3H, t, CH3-CH2CO) . Mass spectra m/z (%): 480 (37) [M+.], 450 (11), 378 (63), 380 (52) and 304 (100). Anal.Calcd. for C27H28O8 (480.18): C, 67.49; H, 5.87, Found: C, 67.45; H, 5.85 |
| Ethyl -4-(4,6,7- trimethoxy benzofuran-5- yl)-6-(4-nitro phenyl) -2-oxo -cyclohexa-3-ene-1-ethyl carboxylate (6c) m.p. 222-224 0C ; yield (78% ); FT-IR: ν (cm-1) 3064 (C-H aromatic), 2943 ( C-H aliphatic), 1577 (C=C), 1723 and 1789 (C=O). 1H-NMR (δ, ppm): 7.89 ( 1H, d, H-2 furan ), 7.52 ( 4H, dd, Ar-H ) , 7.36 ( 1H, d, H-3 furan ), 6.59 (1H, s, CH=C cyclohexenone), 4.23 ( 3H, s, OCH3 ), 4.18 ( 3H, s, OCH3 ), 3.93 ( 3H, s, OCH3 ), 3.64 ( 4H, m, cyclohexenone protons), 3.25 (2H, q, CH2-CO) and 2.24 (3H, t, CH3-CH2CO) . Mass spectra m/z (%): 495 (37) [M+.], 425 (74), 423 (39), 380 (46) and 378 (100). Anal.Calcd. for C26H25NO9 (495.15): C, 63.03; H, 5.09; N, 2.83, Found: C, 63.01; H, 5.06; N, 2.80 |
| IV.7. General procedure of 4-(4,6,7- trimethoxy benzofuran-5- yl)-6-(substituted phenyl)-pyrimidin-2(1H)-one (7a-c) |
| A mixture of chalcones (2a-c) (0.01mol), urea (0.01mol) in 10 ml dry ethanol containing glacial acetic acid (0.5 ml).was refluxed for 6-8 hr. after cooling, the reaction mixture was poured onto ice-water (50 ml). The solid formed was collected by filtration, air dried and crystallized from absolute ethanol to give the title compounds. |
| 4-(4,6,7- trimethoxy benzofuran-5- yl)-6 phenyl-pyrimidin-2(1H)-one (7a) m.p. 233-235 0C ; yield (71% ); FT-IR: ν (cm-1) 3055 (C-H aromatic), 2965 ( C-H aliphatic), 1577 (C=C), 1623 (C=N), 1789 (C=O) and 3399 (NH).. 1H-NMR (δ, ppm): 7.89 ( 1H, d, H-2 furan ), 7.52-7.45 ( 6H, m, Ar-H and pyrimidine proton ) , 7.36 ( 1H, d, H-3 furan ), 4.23 ( 3H, s, OCH3 ), 4.18 ( 3H, s, OCH3 ), 3.93 ( 3H, s, OCH3 ), and 11.3 (1H, s, NH). Mass spectra m/z (%): 378 (67) [M+.], 302 (49), 288 (67) and 208 (100). Anal.Calcd. for C21H18N2O5 (378.38): C, 66.66; H, 4.79; N, 7.40, Found: C, 66.64; H, 4.77; N, 7.38 |
| 4-(4,6,7- trimethoxy benzofuran-5- yl)-6-(4-methoxyphenyl)-pyrimidin-2(1H)-one (7b) m.p. 225-227 0C ; yield (64% ); FT-IR: ν (cm-1) 3063 (C-H aromatic), 2980 ( C-H aliphatic), 1569 (C=C), 1650 (C=N), 1767 (C=O) and 3383 (NH).. 1H-NMR (δ, ppm): 7.97 ( 1H, d, H-2 furan ), 7.59-7.49 ( 5H, m, Ar-H and pyrimidine proton ) , 7.23 ( 1H, d, H-3 furan ), 4.29 ( 3H, s, OCH3 ), 4.18 ( 3H, s, OCH3 ), 3.91 ( 3H, s, OCH3 ), 3.87 ( 3H, s, OCH3 ) and 10.1 (1H, s, NH). Mass spectra m/z (%): 408 (79) [M+.], 378 (73), 364 (59), 302 (29) and 288 (100). Anal.Calcd. for C22H20N2O6 (408.13): C, 64.70; H, 4.94; N, 6.86, Found: C, 64.68; H, 4.91; N, 6.84 |
| 4-(4,6,7- trimethoxy benzofuran-5- yl)-6-(4-nitrophenyl)-pyrimidin-2(1H)-one (7c) m.p. 210-212 0C ; yield (68% ); FT-IR: ν (cm-1) 3077 (C-H aromatic), 2990 ( C-H aliphatic), 1570 (C=C), 1659 (C=N), 1780 (C=O) and 3372 (NH).. 1H-NMR (δ, ppm): 7.91 ( 1H, d, H-2 furan ), 7.61-7.55 ( 5H, m, Ar-H and pyrimidine proton ) , 7.25 ( 1H, d, H-3 furan ), 4.24 ( 3H, s, OCH3 ), 4.15 ( 3H, s, OCH3 ), 3.85 ( 3H, s, OCH3 ), and 9.7 (1H, s, NH). Mass spectra m/z (%): 423 (79) [M+.], 378 (28), 380 (49), 304 (44) and 288 (100). Anal.Calcd. for C21H17N3O7 (423.38): C, 59.57; H, 4.05; N, 9.93, Found: C, 59.55; H, 4.02; N, 9.90 |
| IV.8. General procedure of 4--(substituted phenyl) -6- (4,6,7- trimethoxy benzofuran-5- yl) -1,2,3,4- tetrahydropyrimidin-2-thione (8a-c) |
| A mixture of chalcones (2a-c) (2 mmol), sodium hydroxide (0.2 g in 1 ml water ) in 30 ml ethanol, thiourea (2 mmol) was added. The reaction mixture was refluxed for 3-5 hr. After cooling, the solid formed was collected by filtration, air dried and crystallized from the ethanol to give the title compounds. |
| 4-phenyl-6-(4,6,7-trimethoxybenzofuran-5-yl)-1,2,3,4-tetrahydropyrimidin-2-thione (8a) m.p. 189-1910C ; yield ( 88% ); FT-IR: ν (cm-1) 2180 (C-H aromatic), 1976 (C-H aliphatic), 1559 (C=C),1250 (C=S), 3390 and 3415 (NH). 1H-NMR (δ, ppm): 7.98 ( 1H, d, H-2 furan ), 7.65 ( 5H, m, Ar-H) , 7.25 ( 1H, d, H-3 furan ), 7.0-6.7 (2H, 2d, CH=CH), 4.24 ( 3H, s, OCH3 ), 4.15 ( 3H, s, OCH3 ), 3.85 ( 3H, s, OCH3 ), 11.3 (1H, s, NH) and 9.7 (1H, s, NH). Mass spectra m/z (%): 396 (13) [M+.], 320 (35), 290 (28), 260 (62) and 230 (100). Anal.Calcd. for C21H20N2O4S (396.46): C, 63.62; H, 5.08; N, 7.07; S, 8.09 Found: C, 63.60; H, 5.06; N, 7.05; S, 8.07 |
| 4--(4-methoxyphenyl) -6- (4,6,7- trimethoxy benzofuran-5- yl) -1,2,3,4-tetrahydropyrimidin-2-thione (8b) m.p. 210-1120C ; yield ( 81% ); FT-IR: ν (cm-1) 2176 (C-H aromatic), 1985 (C-H aliphatic), 1544 (C=C), 1245 (C=S), 3377 and 3435 (NH). 1H-NMR (δ, ppm): 7.94 ( 1H, d, H-2 furan ), 7.62 ( 4H, dd, Ar-H) , 7.19 ( 1H, d, H-3 furan ), 7.1-6.68 (2H, 2d, CH=CH), 4.24 ( 3H, s, OCH3 ), 4.15 ( 3H, s, OCH3 ), 3.85 ( 3H, s, OCH3 ), 3.81 ( 3H, s, OCH3 ), 11.3 (1H, s, NH) and 9.7 (1H, s, NH). Mass spectra m/z (%): 426 (39) [M+.], 396 (23), 366 (56), 336 (37) and 230 (100). Anal.Calcd. for C22H22N2O5S (426.49): C, 61.96; H, 5.20; N, 6.57; S, 7.52 Found: C, 61.95; H, 5.18; N, 6.55; S, 7.50 |
| 4--(4-nitrophenyl) -6- (4,6,7- trimethoxy benzofuran-5- yl) -1,2,3,4-tetrahydropyrimidin-2-thione (8c) m.p. 222-224 0C ; yield ( 78% ); FT-IR: ν (cm-1) 2055 (C-H aromatic), 1970 (C-H aliphatic), 1536 (C=C),1248 (C=S), 3359 and 3426 (NH). 1H-NMR (δ, ppm): 7.99 ( 1H, d, H-2 furan ), 7.66 ( 4H, m, Ar-H) , 7.21 ( 1H, d, H-3 furan ),7.15-6.67 (2H, 2d, CH=CH), 4.22 ( 3H, s, OCH3 ), 4.15 ( 3H, s, OCH3 ), 3.85 ( 3H, s, OCH3 ), 11.3 (1H, s, NH) and 9.7 (1H, s, NH). Mass spectra m/z (%): 441 (31) [M+.], 407 (83), 377 (46), 332 (26) and 196 (100). Anal.Calcd. for C21H19N3O6S (441.46): C, 57.13; H, 4.34; N, 9.52; S, 7.26 Found: C, 57.10; H, 4.32; N, 9.50; S, 7.24 |
| IV.9. General procedure of 5-(6-(substituted phenyl)-2,3-dihydro-6H-thiazino[3,2-a]-pyrimidine-4-one-8-yl)- 4,6,7-trimethoxy benzofuran (9a-c) A mixture of (8a-c) (2mmol), bromopropionic acid (2 mmol) in a mixture of glacial acetic acid (30 ml) and acetic anhydride (10ml) in the presence of anhydrous sodium acetate (0.5 g) was refluxed f0r 5-7 hr's. the reaction mixture was cooled and poured onto cold water with stirring, the solid formed was filtered off and crystallized from ethanol to give the title compounds. |
| 5-(6-phenyl-2,3-dihydro-6H-thiazino[3,2-a]-pyrimidine-4-one-8-yl)-4,6,7-trimethoxy benzofuran (9a) m.p. 235-237 0C ; yield ( 71% ); FT-IR: ν (cm-1) 3074 (C-H aromatic), 2980 ( C-H aliphatic), 2560 (C=C), 1660 (C=N) and 1755 (C=O) . 1H-NMR (δ, ppm): 7.97 ( 1H, d, H-2 furan ), 7.60-7.32 ( 6H, m, Ar-H and Hb of pyrimidine) , 7.19 ( 1H, d, H-3 furan ), 5.7 (1H, d, Ha pyrimidine), 3.4-3.55 (4H, m, 2CH2 thiazine protons), 4.24 ( 3H, s, OCH3 ), 4.15 ( 3H, s, OCH3 ) and 3.85 ( 3H, s, OCH3 ). Mass spectra m/z (%): 450 (21) [M+.], 374 (34), 344 (65), 314 (61) and 228 (100). Anal.Calcd. for C24H22N2O5S (450.51): C, 63.98; H, 4.92; N, 6.22; S, 7.12 Found: C, 63.96; H, 4.90; N, 6.20; S, 7.10 |
| 5-(6-(p-methoxyphenyl)-2,3-dihydro-6H-thiazino[3,2-a]-pyrimidine-4-one-8-yl)-4,6,7-trimethoxy benzofuran (9b) m.p. 241-243 0C ; yield ( 75% ); FT-IR: ν (cm-1) 3066 (C-H aromatic), 2959 ( C-H aliphatic), 2555 (C=C), 1687 (C=N) and 1780 (C=O) . 1H-NMR (δ, ppm): 7.88 ( 1H, d, H-2 furan ), 7.59-7.39 ( 5H, m, Ar-H and Hb of pyrimidine) , 7.17 ( 1H, d, H-3 furan ), 5.5 (1H, d, Ha pyrimidine), 3.50-3.55 (4H, m, 2CH2 thiazine protons), 4.21 ( 3H, s, OCH3 ), 3.99 ( 3H, s, OCH3 ), 3.8 ( 3H, s, OCH3 ) and 3.87 ( 3H, s, OCH3). Mass spectra m/z (%): 480 (35) [M+.], 450 (36), 420 (72), 314 (48) and 284 (100). Anal.Calcd. for C25H24N2O6S (480.53): C, 62.49; H, 5.03; N, 5.83; S, 6.67 Found: C, 62.47; H, 5.01; N, 5.81; S, 6.65 |
| 5-(6-(p-nitrophenyl)-2,3-dihydro-6H-thiazino[3,2-a]-pyrimidine-4-one-8-yl)-4,6,7-trimethoxy benzofuran (9c) m.p. 241-243 0C ; yield ( 70% ); FT-IR: ν (cm-1) 3077 (C-H aromatic), 2967 ( C-H aliphatic), 2558 (C=C), 1684 (C=N) and 1773 (C=O) . 1H-NMR (δ, ppm): 7.89 ( 1H, d, H-2 furan ), 7.58-7.41 ( 5H, m, Ar-H and Hb of pyrimidine) , 7.17 ( 1H, d, H-3 furan ), 5.5 (1H, d, Ha pyrimidine), 3.49-3.56 (4H, m, 2CH2 thiazine protons), 4.21 ( 3H, s, OCH3 ), 4.11 ( 3H, s, OCH3 ) and 3.80 ( 3H, s, OCH3 ). Mass spectra m/z (%): 495 (19) [M+.], 465 (49), 435 (62), 284 (29) and 118 (100). Anal.Calcd. for C24H21N3O7S (495.50): C, 58.17; H, 4.27; N, 8.48; S, 6.47 Found: C, 58.15; H, 4.25; N, 8.45; S, 6.45 |
| IV.10. General procedure General procedure of 5-(5-(substituted phenyl)-2,3-dihydro-6H-thiazolo[3,2-a]- pyrimidin-3-one-7-yl)-4,6,7-trimethoxy benzofuran (10a-c) A mixture of (8a-c) (2mmol), chloroacetic acid (2mmol) in a mixture of glacial acetic acid (30 ml) and acetic anhydride (10ml) in the presence of anhydrous sodium acetate (0.5 g) was refluxed for 5-7 hr's. the reaction mixture was cooled and poured onto cold water with stirring, the solid formed was filtered off and crystallized from the proper solvent to give the title compounds. |
| 5-(5-phenyl)-2,3-dihydro-6H-thiazolo[3,2-a]-pyrimidin-3-one-7-yl)-4,6,7-trimethoxy benzofuran (10a) m.p. 239-241 0C ; yield (77% ); FT-IR: ν (cm-1) 3086 (C-H aromatic), 2972 ( C-H aliphatic), 1570 (C=C), 1662 (C=N) and 1784 (C=O) . 1H-NMR (δ, ppm): 8.01 ( 1H, d, H-2 furan ), 7.61-7.33 ( 6H, m, Ar-H and Hb of pyrimidine), 7.23 ( 1H, d, H-3 furan ), 5.65 (1H, d, Ha pyrimidine), 3.53 (2H, s, CH2 thiazine protons), 4.20 ( 3H, s, OCH3 ), 4.14 ( 3H, s, OCH3 ) and 3.84 ( 3H, s, OCH3). Mass spectra m/z (%): 436 (29) [M+.], 406 (28), 376 (54), 346 (56) and 196 (100). Anal.Calcd. for C23H20N2O5S (436.48): C, 63.29; H, 4.62; N, 6.42; S, 7.35, Found: C, 63.28; H, 4.60; N, 6.40; S, 7.33 |
| 5-(5-(p-methoxyphenyl)-2,3-dihydro-6H-thiazolo[3,2-a]-pyrimidin-3-one-7-yl)-4,6,7-trimethoxy benzofuran (10b) m.p. 255-257 0C ; yield (71% ); FT-IR: ν (cm-1) 3072 (C-H aromatic), 2981 ( C-H aliphatic), 1555 (C=C), 1673 (C=N) and 1773 (C=O) . 1H-NMR (δ, ppm): 8.13 ( 1H, d, H-2 furan ), 7.66-7.40 ( 5H, m, Ar-H and Hb of pyrimidine), 7.19 ( 1H, d, H-3 furan ), 5.75 (1H, d, Ha pyrimidine), 3.61 (2H, s, CH2 thiazine protons), 4.19 ( 3H, s, OCH3 ), 4.17 ( 3H, s, OCH3 ), 4.05 ( 3H, s, OCH3) and 3.97 ( 3H, s, OCH3).Mass spectra m/z (%): 466 (46) [M+.], 436 (37), 406 (63), 346 (49) and 270 (100). Anal.Calcd. for C24H22N2O6S (466.51): C, 61.79; H, 4.75; N, 6.00; S, 6.87, Found: C, 61.77; H, 4.73; N, 6.01; S, 6.85 |
| 5-(5-(p-nitrophenyl)-2,3-dihydro-6H-thiazolo[3,2-a]-pyrimidin-3-one-7-yl)-4,6,7-trimethoxy benzofuran (10c) m.p. 261-263 0C ; yield (71% ); FT-IR: ν (cm-1) 3088 (C-H aromatic), 2977 ( C-H aliphatic), 1546 (C=C), 1666 (C=N) and 1759 (C=O) . 1H-NMR (δ, ppm): 7.99 ( 1H, d, H-2 furan ), 7.81-7.55 ( 5H, m, Ar-H and Hb of pyrimidine), 7.2 ( 1H, d, H-3 furan ), 5.69 (1H, d, Ha pyrimidine), 3.63 (2H, s, CH2 thiazine protons), 4.16 ( 3H, s, OCH3 ), 4.11 ( 3H, s, OCH3 ) and 3.97 ( 3H, s, OCH3).Mass spectra m/z (%): 481 (59) [M+.], 451(37), 421(46), 391 (64) and 346 (100). Anal.Calcd. for C23H19N3O7S (481.48): C, 57.37; H, 3.98; N, 8.73; S, 6.66, Found: C, 57.35; H, 3.96; N, 8.70; S, 6.64 |
| IV.11. General procedure of 2-substituted benzylidine -5-(substituted phenyl)-2,3-dihydro-5H-thiazolo[3,2-a]- pyrimidin-3-one-7-yl)-4,6,7-trimethoxybenzofuran (11a-c) |
| Method A: |
| A mixture of compound (8a-c) (2 mmol), chloroacetic acid (0.19, 2 mmol), sodium acetate anhydrous (0.5 g) in glacial acetic acid (30 ml), acetic anhydride (10 ml) and the appropriate aldehyde (2 mmol) was refluxed for 5-7 hr's. the reaction mixture was cooled and poured onto ice-water. The obtained solid was collected by filtration and crystallized from the proper solvent to give the title compounds. |
| Method B: |
| A mixture of compound (10a-c) (2 mmol), the appropriate aldehyde (2 mmol), acetic acid (30 ml) and acetic acid anhydride (10 ml) was refluxed for 2-3 hr., allowed to cool, then poured onto water the solid formed was collected by filtration and crystallized from the proper solvent to give the title compounds. The obtained products from method A and B were identified by m.p., mixed m.p. and TLC, but method A gives better yield than method B . |
| 2- benzylidine -5- phenyl-2,3-dihydro-5H-thiazolo[3,2-a]-pyrimidin-3-one-7-yl)-4,6,7-trimethoxybenzofuran (11a) |
| m.p. 255-257 0C ; yield (67% ); FT-IR: ν (cm-1) 3077 (C-H aromatic), 2982 ( C-H aliphatic), 1569 (C=C), 1672 (C=N) and 1769 (C=O) . 1H-NMR (δ, ppm): 8.03 ( 1H, d, H-2 furan ), 7.61-7.33 ( 11H, m, Ar-H and Hb of pyrimidine), 7.25 ( 1H, d, H-3 furan ), 5.62 (1H, d, Ha pyrimidine), 3.50 (1H, s, benzylidine protons), 4.20 ( 3H, s, OCH3 ), 4.14 ( 3H, s, OCH3 ) and 3.84 ( 3H, s, OCH3). Mass spectra m/z (%): 524 (54) [M+.], 464 (83), 376 (45), 300 (62) and 270 (100). Anal.Calcd. for C30H24N2O5S (524.59): C, 68.69; H, 4.61; N, 5.34; S, 6.11, Found: C, 68.67; H, 4.60; N, 5.32; S, 6.10 |
| 2- (p-methoxybenzylidine) -5- phenyl-2,3-dihydro-5H-thiazolo[3,2-a]-pyrimidin-3-one-7-yl)-4,6,7- trimethoxybenzofuran (11b) |
| m.p. 269-271 0C ; yield (61% ); FT-IR: ν (cm-1) 3049 (C-H aromatic), 2961 ( C-H aliphatic), 1583 (C=C), 1663 (C=N) and 1740 (C=O) . 1H-NMR (δ, ppm): 7.94 ( 1H, d, H-2 furan ), 7.66-7.31 ( 9H, m, Ar-H and Hb of pyrimidine), 7.19 ( 1H, d, H-3 furan ), 5.99 (1H, d, Ha pyrimidine), 3.48 (1H, s, benzylidine protons), 4.18 ( 6H, s, 2OCH3 ), 4.14 ( 3H, s, OCH3 ), 4.01( 3H, s, OCH3 ) and 3.84 ( 3H, s, OCH3).Mass spectra m/z (%): 584 (53) [M+.], 524 (82), 434 (49), 346 (23) and 208 (100). Anal.Calcd. for C32H28N2O7S (584.64): C, 65.74; H, 4.83; N, 4.79; S, 5.48, Found: C, 65.71; H, 4.80; N, 4.75; S, 5.45 |
| 2- (p-nitrobenzylidine) -5- phenyl-2,3-dihydro-5H-thiazolo[3,2-a]-pyrimidin-3-one-7-yl)-4,6,7- trimethoxybenzofuran (11c) m.p. 279-281 0C ; yield (65% ); FT-IR: ν (cm-1) 3055 (C-H aromatic), 2969 ( C-H aliphatic), 1579 (C=C), 1683 (C=N) and 17952 (C=O) . 1H-NMR (δ, ppm): 7.88 ( 1H, d, H-2 furan ), 7.7-7.37 ( 9H, m, Ar-H and Hb of pyrimidine), 7.21 ( 1H, d, H-3 furan ), 5.73 (1H, d, Ha pyrimidine), 3.51 (1H, s, benzylidine protons), 4.16 ( 3H, s, OCH3 ), 4.14 ( 3H, s, OCH3 ) and 3.92 ( 3H, s, OCH3). Mass spectra m/z (%): 614 (27) [M+.], 554 (78), 524 (49), 434 (73) and 118 (100). Anal.Calcd. for C30H22N4O9S (614.58): C, 58.63; H, 3.61; N, 9.12; S, 5.22, Found: C, 58.60; H, 3.60; N, 9.10; S, 5.20 |
V. CONCLUSION |
| From the above studies it can be concluded that the synthesized compounds exhibit significant antibacterial activity against pathogenic bacteria. Further studies are needed to determine the mode of action of the studied compounds. |
ACKNOWELDEMENT |
| The authors gratefully acknowledge Chemistry Department, Faculty of Science, Taif University, KSA for all facilities provided in terms of the use of the available chemicals and equipment. Also, we would like to thank the Micro analytical center, Cairo University, Cairo, Egypt, for the spectral analysis of the compounds used in this study. |
References |
|