e-ISSN: 2319-9849
e-ISSN: 2319-9849
Inorganic Chemistry Unit, Department of Chemistry, University of Ibadan, Ibadan, Nigeria
Received: 22/08/2013; Revised: 16/10/2013; Accepted: 22/10/2013
Visit for more related articles at Research & Reviews: Journal of Chemistry
Mixed ligand complexes of Riboflavin (L) and 2,2’-Bipyridine (L1) with Mn(II), Fe(II), Co(II), Ni(II), Cu(II) and Zn(II) ions were synthesized and characterized by, infrared and electronic spectroscopies, room temperature magnetic moments, melting points and conductance measurements. The % metal analysis confirmed that the complexes analyzed as [MX2(L)(L1)] where X = Cl/(CH3CO2)/SO4. Infrared spectra data confirmed that coordination is via the imine nitrogen and carbonyl oxygen atoms of the riboflavin, and the nitrogen atoms of the 2,2’-bipyridine molecules respectively. The room temperature magnetic moment and electronic spectra data indicated that all the metal(II) complexes were octahedral, and the Mn(II), Fe(II), Co(II) and Ni(II) complexes showed high spin low spin octahedral equilibrium. The conductance measurements of all the metal(II) complexes in water and DMSO showed that the complexes were all covalent. Interestingly, the in-vitro antibacterial studies of these metal(II) complexes, riboflavin and 2,2’-bipyridine against Bacillus cereus, Escherichia coli, Proteus mirabilis, Pseudomonas aeruginosa, Klebsiella oxytoca and Staphylococcus aureus showed that none of the bacteria was sensitive to the former two compounds, with the exception of Proteus mirabilis which had activities of 20.0 mm and 13.0 mm against the Cu(II) complex and riboflavin. In contrast, all the bacteria were sensitive to 2, 2’-bipyridine, just like Augmentine, although with higher inhibitory zones range of 24.0-47.0 mm proving its potential as a broad spectrum antibacterial agent.
antibacterial, bipyridine, covalent, equilibrium, riboflavin
Mixed-ligand complexes containing nitrogen and oxygen atoms are of significant importance owing to their antimicrobial and anticancer activities [1-3] which are sometimes more effective than that of the free ligands. Similarly, the coordination chemistry of mixed ligand complexes provide new compounds that can act as an active catalyst in reactions of industrial importance that includes hydrogenation, hydroformylation, and oxidative hydrolysis of olefins and carboxylation of methanol [4]. On the other hand, Riboflavin, vitamin B2, is a water-soluble vitamin. Its metabolism is controlled by different hormones which regulate its conversion in flavinadenine dinucleotide and flavin mononucleotide [5]. These two coenzymes catalyze many oxidation-reduction reactions and are essential for production of energy [6,7]. Consequently, Riboflavin has been used in several clinical and therapeutic situations, such as phototherapy treatment of neonatal jaundice, prevention of migraine and together with UV lights it’s effective for inactivating pathogens in platelets and plasma [8].
Furthermore, Riboflavin can be described as a biological chelating ligand due to the existence of nitrogen and oxygen atoms on its structure that can act as coordinating sites for metal ion chelation. The geometric and electronic properties of Riboflavin metal complexes have not been fully exploited by researchers as shown from literature search [6-10]. The choice of 2, 2’-bipyridine as a secondary ligand was due to its versatile roles as building blocks for the synthesis of metallo-dendrimers and as molecular scaffolding for supramolecular assemblies [11].
Thus, our aims are to synthesize the metal(II) complexes of mixed ligand Riboflavin and 2,2 bipyridine, study their coordination properties as well as their in-vitro antibacterial properties for broad-spectrum activity against pathogenic organisms. This is a continuation of our research on the search for biologically active compounds that would serve as lead compounds in drug research [12,13,14,15].
Reagent grade Riboflavin, 2, 2’-bipyridine, Copper(II)chloride tetrahydrate, Nickel(II) chloride hexahydrate, Cobalt(II)chloride hexahydrate, Manganese(II) chloride tetrahydrate, Zinc(II) acetate dihydrate, and Iron(II) sulphate heptahydrate were obtained from Aldrich and BDH chemicals, and were used as received, and solvents were purified by distillation.
Preparation of [Co(L)(L1)Cl2]·3H2OThis complex was prepared by the addition of 0.38 g (1.59 x 10-3 moles) of CoCl2·6H2O to a stirring solution of 1.59 x 10-3 moles (0.60 g, riboflavin) and 1.59 x 10-3 moles (0.25g, 2,2’-bipyridine) in 20 mL of methanol. The resulting homogeneous solution was then refluxed for 3 hours during the products formed. The light orange precipitate obtained was filtered, washed with methanol and dried over silica gel. The same method was used for the preparation of the Mn(II), Fe(II), Co(II), Ni(II), Cu(II) and Zn(II) complexes from their chloride, sulphate and acetate salts respectively.
Physical measurementThe solid reflectance spectra of the complexes were recorded on a Perkin-Elmer λ25 spectrophotometer and infrared spectra were recorded as KBr disc on a Perkin-Elmer FT-IR spectrum BX spectrometer in the range 4000-400 cm-1. The room temperature magnetic susceptibilities at 303K were measured on Sherwood Susceptibility Balance MSB Mark 1. Melting points were determined with Mel-Temp electrothermal machine, and molar conductivity measurements of 1 x 10-3 M solutions in water and DMSO respectively were obtained using electrochemical analyzer Consort C933.
Antibacterial assayThe antimicrobial activities of the synthesized compounds as well as their free ligands were studied using the agar diffusion technique. The bacterial used were identified laboratory strains of Bacillus cereus, Escherichia coli, Proteus mirabilis, Pseudomonas aeruginosa, Klebsiella oxytoca and Staphylococcus aureus. The surface of the agar in a petri dish was uniformly inoculated with 0.2 mL of 18 hour old test bacterial culture. Using a sterile cork borer, 9 mm wells were bored into the agar. Then 0.06 mL of 10 mg/mL concentration of each metal complex in DMSO was introduced into the wells and the plates were allowed to stand on the bench for 30 minutes before incubation at 370C for 24 hours after which inhibitory zones (in mm) were taken as a measure of antibacterial activity. The experiments were conducted in duplicates and Augmentine was used as the reference drug.
The reaction of the Riboflavin (L), 2,2’-bipyridine (L1) with the metal(II) chlorides (Mn, Ni, Cu and Co), FeSO4.7H2O and Zn(CH3COO)2 gave coloured complexes in moderate good yields according to equations 1- 4.
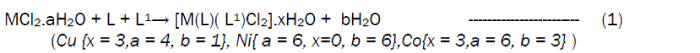
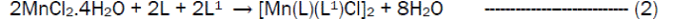
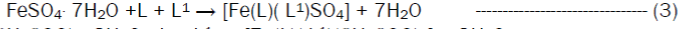
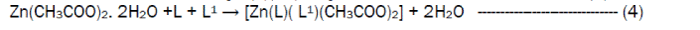
The formation of the metal complexes was confirmed by % metal and distinct decomposition temperature. The ligands, riboflavin (L) and 2, 2’-bipyridine (L1) melted at 280-290 and 70-730C respectively, whereas their metal complexes mostly decomposed in the range 202-2480C, confirming coordination. The complexes were all slightly soluble in methanol, ethanol, nitromethane, and methylene chloride but are all soluble in water with the exception of the Fe(II) complex which was the only complex that dissolved in DMSO. Attempts to isolate suitable single crystal of the metal complexes for X-ray diffraction studies have not been successful till now. Hence % metal, magnetic and spectroscopic data were used to propose possible structures. The analytical data, colours, % metal, melting points, molar conductivity and room temperature magnetic moments for the complexes are presented in Table 1 and the proposed structure is given in Figure 1.
Conductance measurementsThe molar conductance values of the complexes in DMSO and water were in the range 5.26 – 47.8 Ω- 1cm2mol-1 indicating their non-electrolytic nature [16].
Electronic Spectra and Magnetic momentsThe ultraviolet spectra of the compounds were characterized by strong absorption maxima between 25.58 – 26.67 kK and 32.89 - 38.61 kK assigned to n→π* and π→π* respectively (Table 2). The Mn(II) complex showed two absorption bands at 19.53 kK and 22.0 kK assigned to 6A1g → 4E1g and 2T2g → 2T1g transitions consistent with a high spin and low spin octahedral geometry. The effective magnetic moment of high spin Mn(II) complexes are expected to be close to the spin-only value of 5.90 B.M. since the ground term is 6A1g and thus, there is no orbital contribution, whereas low spin octahedral Mn(II) have moments of about 2.0 B.M. Consequently, an observed moment of 4.97 B.M was indicative of spin equilibrium between the high spin and low spin octahedral geometry [17].
The Fe(II) complex had two absorption bands at 17.57 and 19.82 kK typical of 6-coordinate, high spin and low spin octahedral geometry and were assigned to 5T2g → 5Eg and 1A1g → 1T2g transitions. A moment of 5.0-5.5 B.M is usually expected for high spin complex and low spin octahedral Fe(II) complexes are expected to be diamagnetic. In this study, a moment of 3.66 B.M was observed for this complex, which was indicative of spin equilibrium between the high spin and low spin octahedral geometry [18,19].
Similarly, the Co(II) complex gave two absorption bands at 19.89 and 21.98 kK consistent with high and low spin octahedral geometry and were assigned to 4T1g(F) → 4T1g(P) and 2A1g→2T1g transitions. Moments of 4.7- 5.2 and 2.0-2.9 B.M. are expected for high spin and low spin octahedral complexes respectively. However, a magnetic moment of 3.15 B.M was observed which was intermediate between these two values, confirming equilibrium between the high spin octahedral and low spin octahedral geometry [20,21].
In the same vein, the Ni(II) complex showed two absorption bands at 19.65 and 23.0 kK typical of six coordinate high spin and low spin octahedral geometry. These were assigned to 3A2g → 3T1g(P) and 1A1g → 1B1g transitions. Room temperature magnetic moments in the range 2.8-3.3 B.M is expected for high spin octahedral complexes Ni(II) while low spin octahedral Ni(II) complexes have moments in the range 0.78-1.68 B.M. However, an observed moment of 2.1 B.M for this complex, corroborates equilibrium between the high spin octahedral and low spin octahedral geometry [22,23].
The Cu(II) complex exhibited an absorption band at 20.12 kK assigned to 2Eg → 2T2g transition of an octahedral geometry. A moment in the range 1.9–2.2 B.M. is usually observed for mononuclear copper(II) complexes, regardless of stereochemistry, expectedly higher than the spin only moment due to orbital contribution and spin-orbit coupling. The Cu(II) complex in this study, had a moment of 2.16 B.M which was complimentary of octahedral geometry [24].
The Zn(II) complex showed M→L CT transitions at 19.67 and 21.10 kK, as no d-d transition was expected. The complex was expected to be diamagnetic. However, it was paramagnetic with a magnetic moment of 1.04 B.M due to presence of paramagnetic impurities [25].
Infrared SpectraThe relevant bands are presented in Table 2. The medium band at 3402.21 cm-1 in riboflavin is assigned as υ(NH) band [26]. This band appeared mainly as lone band in the complexes, this confirmed non deprotonation of the amino hydrogen and its coordination to the metal(II) ions. The sharp bands at 1579 and 1578 cm-1 in the ligands were assigned as υC=N stretching vibrations and were shifted to 1584 -1575 cm-1 while the bands at 1728 and 1647 cm-1 in the riboflavin were assigned as υC=O stretching vibrations and were shifted to 1729 – 1727 cm-1 and 1650 – 1641 cm-1 respectively in the spectra of the metal(II) complexes confirming coordination through the nitrogen atoms of the riboflavin and 2,2’-bipyridine ring (Chadar and Khan, 2006) as well as the carbonyl oxygen atom of the riboflavin. Furthermore, the new bands in the range 598 - 502 cm-1, 495-445 cm-1 and 365-350 cm-1 were assigned to υ(M-N), υ(M-O)/ υ(M-S) and υ(M-Cl) respectively due coordination of the N-atoms of the riboflavin and 2,2’-bipyridine; oxygen atoms of the carbonyl, sulphato, acetato ligands; and chlorine atoms in complexation to metal ions [9,10]. Conversely, these bands were absent in the spectra of the riboflavin and 2,2’-bipyridine, confirming coordination in the metal complexes.
Antibacterial activitiesRiboflavin (L) was inactive against all the tested bacteria with the exception of P. mirabilis, with which it had an activity of 13.0 mm. However, 2, 2’-bipyridine (L1) exhibited very good activity against all the tested bacteria with inhibitory zones range of 24.0.0-47.0 mm. On the contrary, their metal complexes were generally inactive, with the exception of the Cu(II) complex, which had an activity of 20 mm against P. mirabilis. The best activity of 2, 2’-bipyridine was attributed to point mutation, which causes alteration of the DNA due to the transformation of the bases in a pair of nucleotides during DNA replication, leading to oxidative damages in DNA [27]. Expectedly, riboflavin was mostly inactive being a growth supplement in humans and microorganisms [7]. Generally, metal(II) complexes are expected to be more effective than the metal-free ligand, due to chelation, which reduces the polarity of the metal atom and increases lipophilic character, favouring its permeation through lipid layers of the bacterial membrane. Thus, the inactivity of these metal complexes may be attributed to their probable lipophobic nature [28]. Interestingly, 2, 2’-bipyridine (24.0-47.0 mm) was more active than the standard antibiotic, Augmentine (21.0-36.0 mm) against all the tested bacteria. Thus, proving its potential as a broad-spectrum antibacterial agent (Table 3).
Mixed ligand complexes of riboflavin (L) and 2,2’-bipyridine (L1) with Mn(II), Fe(II), Co(II), Ni(II), Cu(II) and Zn(II) ions were synthesized and characterized by infrared and electronic spectroscopies, room temperature magnetic moments, melting points and conductance measurements. Electronic spectra and room temperature magnetic moment data corroborated octahedral geometry for all the metal complexes. The conductance measurements in water and DMSO showed that the complexes were all covalent. The in-vitro antibacterial studies of the complexes against B. cereus, E. coli, P. mirabilis, P. aeruginosa, K. oxytoca and S. aureus showed that they were generally inactive.