ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Anuj Kumar Garg1, A. K. Gupta2* and Ashu Rani3*
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
The leaching kinetics of nitrate (NO3 -) in agricultural (pH = 7.2) and non-agricultural soils (pH = 6.8) was studied using undisturbed glass columns method . The soils were taken from the agriculture field and barren land near Dheerkhera agricultural area, Hapur. The Sodium nitrate (NaNO3) is used as a source of NO3 -. Power form equations (LRobs = k1 [NO3 - ]i 2.91 for agricultural and (LRobs = k2 [NO3 - ]i 1.829 for non-agricultural soils have been derived for dependence of LRobs on leachable concentration of NO3 - present initially. The effects of pore volume, temperature, Ca hardness have been observed on leaching rate. Change in water filled porosity θ (cm3cm-3) of the column has been found to affect LRobs. The LRobs is found to increase with temperature in the range 30-50°C. Experimental data fitting on various kinetic models showed first order kinetic models and parabolic diffusion to be most suited for predicting leaching rates.
Keywords |
| Nitrate, adsorption, leaching, glass column, first order, pore volume, BTC. |
INTRODUCTION |
| Nitrogen is both an essential nutrient and a large source of pollution on the terrestrial ecosystems. Due to an important component of plant nutrients, nitrogen plays an important role in increasing crops yields and its quality [1]. Hence the nitrogen in form of Nitrate, Ammonium, or Urea (which rapidly hydrolyses to ammonium) are used to increase the crops yields. In contrast to ammonium ions, NO- 3 are not adsorbed by the negatively charged colloids that dominates most soils. Therefore nitrates ions can move downward freely with the drainage water and are thus readily leached from the soil. Such leaching losses will cause serious environmental problems [1]. |
| Leaching is one of the most important physical processes responsible for migration of soil nutrients and pollutants. Over fertilized agricultural areas poses a threat to the ground water quality mainly because of leaching of salts through macropores along with percolating water [2]. As the water moves through the soil profile, it dissolves additional salts from the soil and transport them to subsurface and ground water [3].The leaching rates of salts are basically the relative mobility of water along with salt movement. One of the worst consequence of leaching from polluted sites as well as from over fertilized agricultural fields during irrigation, rain events and water percolation is contamination of subsurface and ground water [4]. By using the excess of nitrogen fertilizers at the rate higher than plant uptake can increase the chance of nitrate leaching by many times [5]. Leaching of solute also depends on amount of water applied by irrigation or natural precipitation [6] and the amount, timing and species of fertilizer applied [7]. Nitrate leaching from the unsaturated zone is a complex phenomenon involving many factors such as land use practices, soil nitrogen dynamics, on-ground nitrogen loading, groundwater recharge, soil characteristics, the depth to water table [8]. Leaching may depend on macro porosity of soil [9] as well as laminar flow of water in cracks and channels found in soils [10]. Leaching also depends on salt solubility, initial water content of soil [11], pH [12], temperature, etc. |
| Studies conducted in different parts of the world have shown that farmers often use amount of N fertilizers that exceed the N requirement of crops. Nitrate is soluble and negatively charged and thus has a high mobility and potential for loss from the unsaturated zone by leaching [15],[16]. Many studies showed high correlation and association between agriculture and nitrate concentration in groundwater [17],[18],[19]. The extensive use of fertilizers is considered to be a main non-point source of the nitrate [20],[21],[22] and point sources of nitrogen such as septic systems are shown to contribute to nitrate pollution of groundwater [23]. Elevated nitrate concentrations in drinking water can cause Methemoglobinemia in infants and stomach cancer in adults [2],[18]. The US Environmental Protection Agency (US EPA) has established a maximum contaminant level of 10 mg/L NO3 - N . As a significant quantity of nitrogenous fertilizer can be transported through preferential flow pathway in the soil [23], [20]. The downward movement of NO3 - in agricultural and non-agricultural soils is studied using glass column method, The Effect of Ca hardness of extractant, irrigation water quality, water filled porosity of the soil, temperature and salt concentrations have been investigated on rate of leaching of nitrate ion in soil. |
RELATED WORK |
| Literature survey revealed that studies on kinetics of leaching of various chemicals [31,34] through soils and soil mineral are rare. Most of the desorption and leaching [32, 33, 37,38] studies of nitrate give only preliminary idea about the leaching. None of them reported utilization of initial rate kinetic method for studying leaching of chemicals on columns of natural soils with continuous or restricted flow in saturated and unsaturated conditions. The present investigation has been carried out to investigate the leaching kinetics of NaNO3, a highly soluble NO3 - salt present in the agricultural field. Most of the studies conducted on NO3 -leaching are from the regions where rainfall is abundant and well distributed [26] but studies under semi arid condition are scares. |
MATERIALS AND METHODS |
| Soil samples were collected from the agricultural fields of Dheerkhera area of Hapur where crops are grown and chemical fertilizers are continuously applied during the last ten years. Non-agricultural soil samples were collected from barren lands where crops are not grown and chemical fertilizers are not applied. Soil samples were collected by digging a deep pit. Samples were collected and placed in plastic bags and labeled according to their field. Soil samples were collected 2-3 times in a month at every site during the entire study period. These samples were dried and sieved. Table 1 gives physicochemical characteristics of the soil analyzed using standard methods [27]. |
| The leaching kinetics of NaNO3 was studied by determining the nitrate concentrations in the leachates with time. Initial leaching rates (LRobs) have been calculated using plane mirror method [29]. Nitrate was estimated using standard spectrophotometric method [27]. In this method aliquot was evaporated to dryness over a hot water bath, and 2 mL of Phenol disulphonic acid was added to it and then 5 mL Ammonium hydroxide was added to develop colour and diluted to 100 mL with deionized water. The calibration curve was obtained at 410 nm using NO3 - solution of different concentrations. The concentration (mg L-1) in leachates were converted to mg kg-1 in soil during kinetic work. |
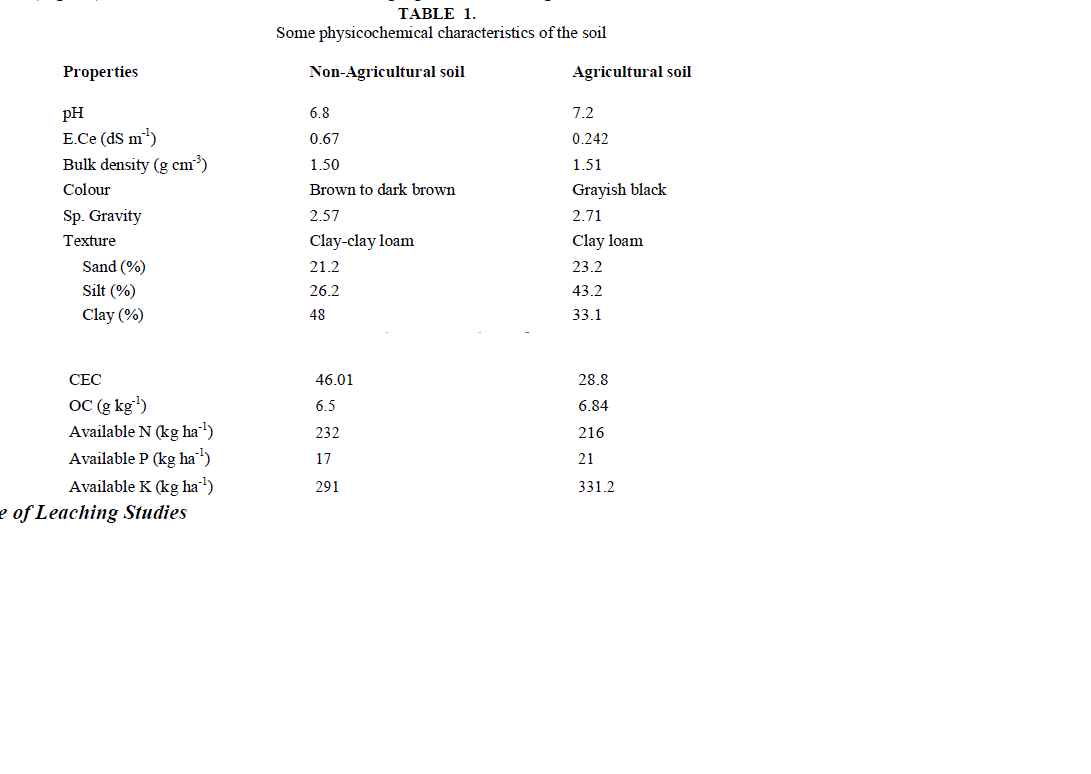 |
 |
RESULTS AND DISCUSSION |
A. Total Leachable Nitrate [NO3 -]i |
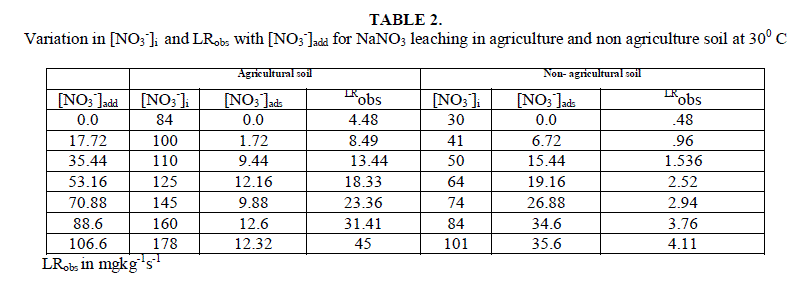
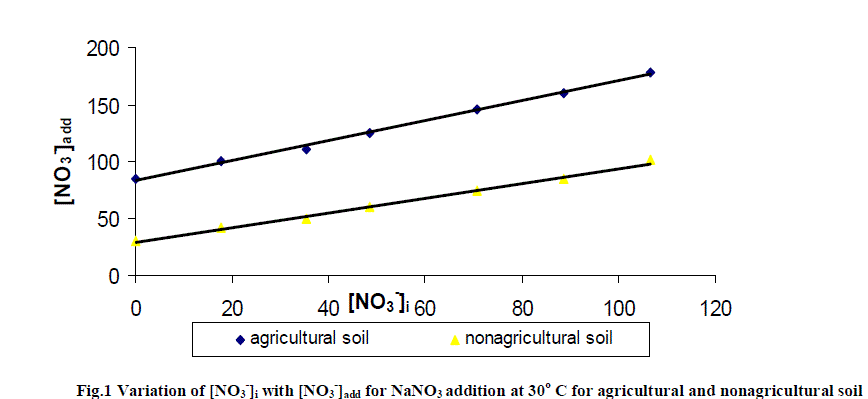
| The soil itself has naturally leachable [NO3 -]s in it. On adding NO3 - from outside in the form of NaNO3 , concentration of leachable nitrate increases, with the increase in amount of added salt. It is clear from the result of table 2 and fig.1 that [NO3 -]s i.e. (leachable nitrate present naturally in soil column) are higher in agricultural soil in comparison to non- agricultural soil. |
 |
B. Leaching of NaNO3 |
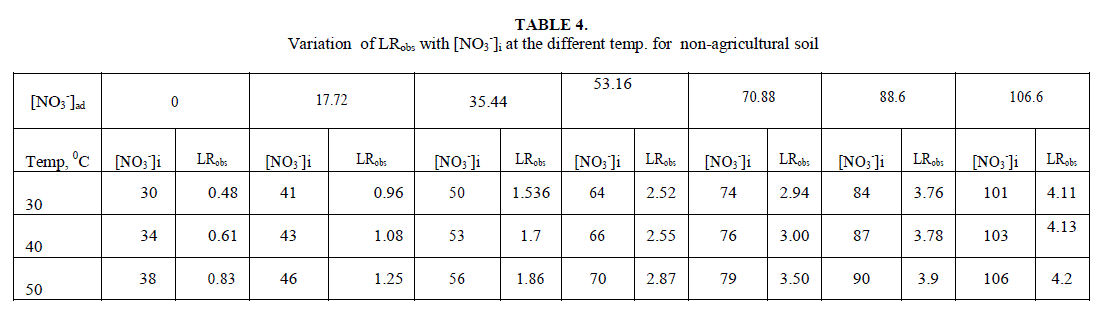
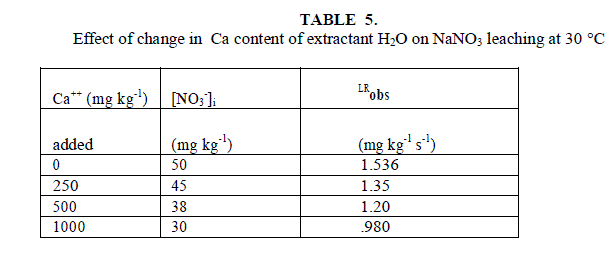
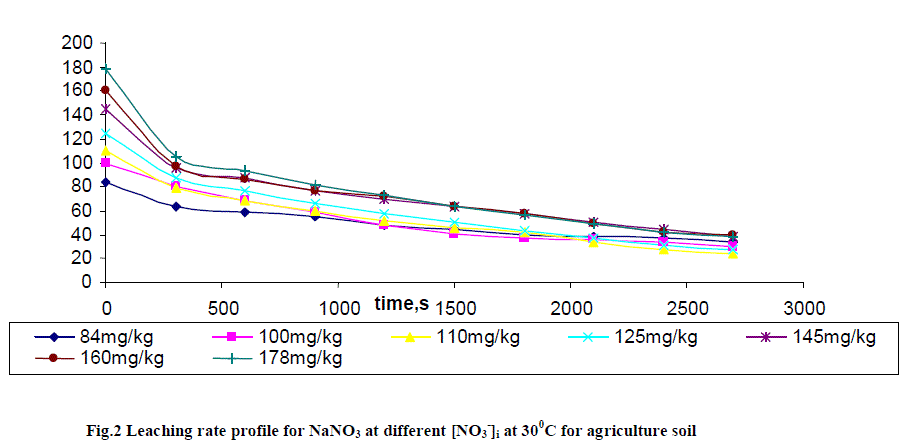
| Leaching of NO3 - ion was studied at different added concentration of NaNO3 on soil columns. Amount of NaNO3 was varied from 0 to 106.6 mg kg-1. The flow rate of percolating water was 10 ± 2 mL per 10 minutes. The results of NaNO3 leaching for variable [NO3 -]i are given in table 2 for agriculture and non-agriculture soil. From the Figs. 2 and 3 it is clear that initial leaching is relatively fast for almost all the [NO3 -]i in both types of soils. The LRobs values are found higher in agriculture soil in comparison to non-agriculture soil for similarly added NaNO3 . |
 |
C. Leaching Rate Profile & Dependence of LRobs on [NO3 -] |
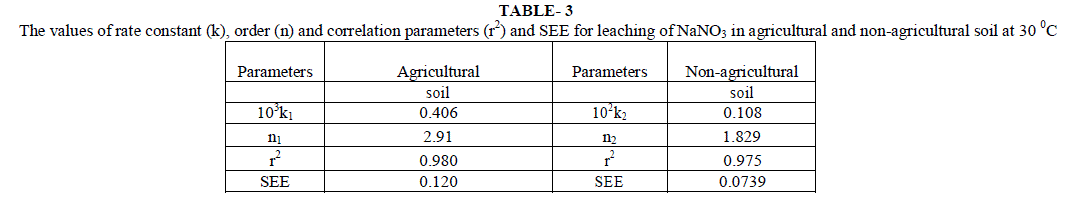
| Initial rates for leaching, LRobs, represents the rate of change in leachable concentrations, [NO3 -]1 with time. LRobs values were obtained from the slope of the initial rate plot using the plane mirror method by Max Latshaw[29].It can be concluded from table 2 that with the increase of [NO3 -]i , the LRobs is also increased. The log–log plot between the initial total leachable content [NO3 -]i and the LRobs for agricultural and non agricultural soil indicated a fractional order of more than one i.e. 2.91 and 1.82 respectively given in table 3. The rate laws are given for NaNO3 for agricultural and non agricultural soil are |
 |
 |
G. APPLICATION OF STUDY |
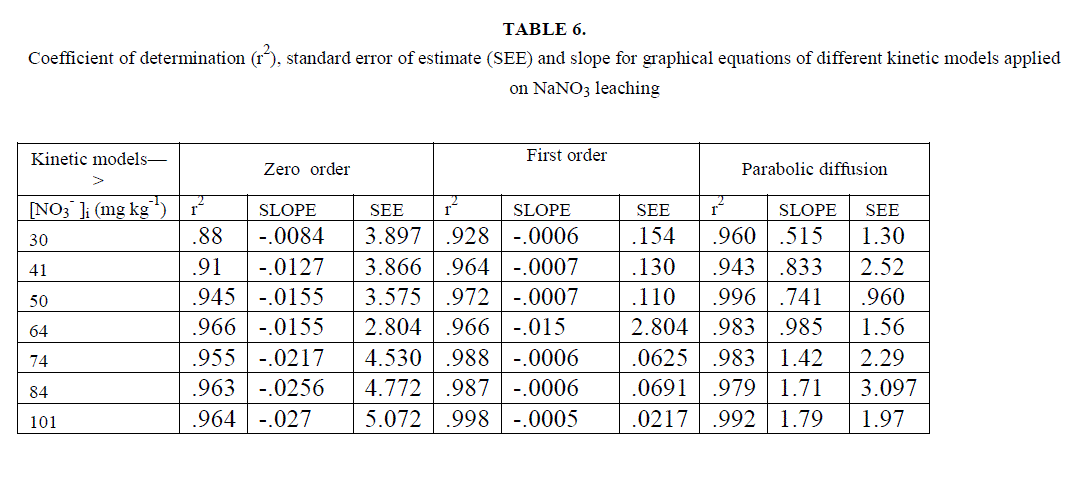
| Most of the earlier studies on kinetics of NO3 -desorption from soil found that release of NO3 - deviate from zero order kinetic reaction and described better by a first order reaction [22]. Ref [36] used the framework of Freundlich equation and developed kinetic model for desorption incorporating power form equation. He explained that parameters of the models depended upon the length of the incubation period allowed, addition of solute to soil and the start of desorption further. Chien and Clayton (1980) described desorption of phosphate by modified form of Elovich equation. Power form equation used by us is different in the manner that it includes initial leaching rates but not the initial amount of desorbable NO3 -. This equation is more suited to present soil conditions. Besides, experimental results were also fitted to the previously explained conventional kinetic models. Average values of slope, r2 and SEE are given in table 6, for different concentration of [NO3 -]i for the NO3 - salts . It is clear from the table that first order equation (Fig. 5) and parabolic diffusion (Fig. 6) are most suitable to describe NO3 - leaching. |
 |
 |
CONCLUSION |
| This study was conducted following a multidisciplinary approach to obtain data necessary for a better representation of NO3 - transport from the soil. It has been successfully represented the results of column study in the power form equation to represent nitrate leaching accurately. A fractional order of >1.5 and < 3.0 has been observed for leaching of NaNO3. Results of this study showed that leachable NO3 - is higher in agriculture soil columns in comparison to non-agricultural soil columns while adsorbed NO3 - concentration is higher in non-agricultural soil columns. Increasing the water filled porosity does not impart any significant change in [NO3 -]i as well as on LRobs . |
| Several earlier studies [31], [33], [34] have given higher levels of nitrate in agricultural fields but relationship between rate of leaching with concentration of nitrate in soil were not reported in any study. This method can also be applied in the agricultural fields to know fertilizer requirement of plants as well as leaching rates below root zone. |
References |
|