ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
| Saurabh Singh1, Manish Jain2 and Amit Pal3 M.Tech. Student, Department of Mechanical Engineering, Delhi Technological University, Delhi, India1 Asst. Prof., Department of Mechanical Engineering, RJIT, Gwalior, India2 Associate Prof., Department of Mechanical Engineering, Delhi Technological University, Delhi, India3 |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Vegetable oils are a suitable alternative to diesel in compression ignition (CI) engines. The use of vegetable oils in a C I engine results in low CO, HC and smoke opacity emissions compared to a conventional diesel fuel. Biodiesel, a clean renewable fuel, has recently been considered as the best substitute for a diesel fuel because it can be used in any CI engine without the need for modification. Chemically, biodiesel is a mixture of methyl esters with long chain fatty acids and is typically made from non-toxic, biodiesel resources such as vegetable oils (Jatropha, Karanja, Thumba etc.), animal fats or even waste cooking oils (WCO). Biodiesel processing is required to refine the vegetable oil feedstock and convert it into biodiesel, so as to meet the desired C I engines fuels specifications. This paper describes the basic processing required for the vegetable oil feedstock to make it usable in C I engines
Keywords |
| Biodiesel, Renewable fuel, Smoke opacity, Engine emission. |
INTRODUCTION |
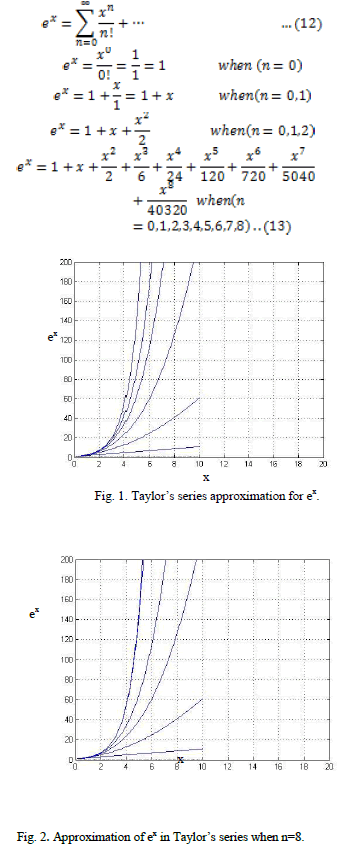
| With ever increasing energy demand and depleting fossil fuel reserves, bio fuels are increasingly being considered as the energy option for the future. The world is presently confronted with the twin crises, one is fossil fuel depletion and the other is environment degradation. Indiscrimination extraction and lavish consumption of fossil fuels have led to reduction in underground based carbon resources. According to an estimate, the reserves will last 218 yrs for coal, 41 yrs for oil and 63 yrs for natural gas. Many researchers around the world have explored several alternative energy resources, like biogas, biomass, alcohol, hydrogen, vegetable oil, which have the potential to quench the everincreasing energy thirst of today’s population[1]. For the last century, petroleum derived fuels have been the major source of the world’s energy. However, it is predicted that fossil fuel will be depleted in the near future. Future projections indicate that economics and energy needs will increase the focus on the production of synthetic fuels derived from non-petroleum sources, including biomass and waste products among others [2]. |
| The idea of using vegetable oil as a substitute for diesel fuel was demonstrated by the inventor of the diesel engine, Rudolph Diesel, around the year 1900[3]. Various derivatives such as micro emulsions or blends of various vegetable oils with conventional fuel have been proposed as alternative fuels for diesel engines. The esters of vegetable oils or animal fats appear to be the most promising alternative. Today, methyl or ethyl esters of fatty acids are used as substitute to petroleum-based diesel fuel under the name of “biodiesel”. Bio-diesel fuels have many advantages over petroleum diesel fuel: produce less smoke and particles, have higher cetane number, produce lower carbon monoxide and hydrocarbon emissions, are renewable, biodegradable and non-toxic. |
| The process of conversion of vegetable oil into biodiesel is completed by maintaining the reaction stoichiometrically, 3:1 to 9:1 molar ratio of alcohol to triglyceride is necessary. Due to the fact that the transesterification is an equilibrium reaction, an excess of alcohol is used to displace the reaction towards esters formation. Fats and alcohols are not totally miscible, so their reaction takes place at the interface and it is a very slow process. The reaction is catalyzed by alkali, acid or enzyme. Enzymes-catalyzed procedures, using lipase as catalyst, do not produce side reactions [4-5], but the lipases are very expensive for industrial scale production and a three-step process was required to achieve a 95% conversion. Acid-catalyzed process is useful when a high amount of free acids are present in the vegetable oil [6], but the reaction time is very long (48–96 h), even at the boiling point of the alcohol, and a high molar ratio of alcohol was needed (20:1 wt/wt to the oil). |
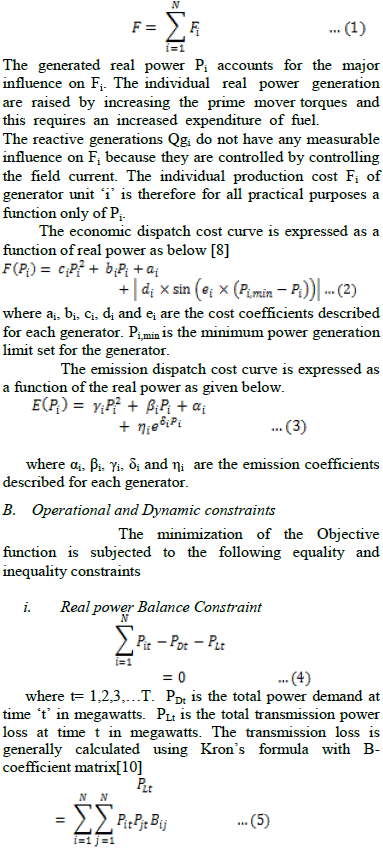
| Biodiesel can be used neat, as a pure fuel it is known as B100. However, it is often blended with petroleum-based diesel fuel and the blend is designated “BXX” where XX is the percentage of biodiesel in the blend. For example, B20 is a blend of 20% biodiesel and 80% petroleum diesel fuel. |
II. PROCESS OF BIO-DIESEL PRODUCTION |
| A. Transesterification Reaction |
| Transesterification process also called alcoholysis is the displacement of alcohol from an ester by another alcohol in a process similar to hydrolysis except that an alcohol is used instead of water. This has been widely used to reduce the viscosity of the triglycerides. The transesterification process in general is represented as: |
 |
| If methanol is used in this process then it is called methanolysis. Methanolysis of triglycerides is represented as |
 |
| Transesterification is one of the reversible reactions and proceeds essentially by mixing the reactants. However, the presence of a catalyst (a strong acid or base) accelerates the conversion. |
| Virtually all commercial biodiesel producers use an alkali-catalyzed process for the transesterification process, other approaches have been proposed including acid catalysis [7] and enzymes [8-11]. The use of acid catalysts has been found to be useful for pretreating high free fatty acid feedstock but the reaction rates for converting triglycerides to methyl esters are very slow. Enzymes have shown good tolerance for the free fatty acid level of the feedstock but the enzymes are expensive and unable to provide the degree of reaction completion required to meet the ASTM fuel specification [12]. Immobilization of the enzyme and use of multiple enzymes in sequence may provide future opportunities in this area [9-11]. |
| B. Alkali-catalyzed Transesterification |
| The processes involved in biodiesel production from feedstock containing low level of free fatty acids (FFA). Alcohol, catalyst, and oil are combined in a reactor and agitated for approximately one hour at 60ºC. The glycerol is removed from the methyl esters, due to the low solubility of glycerol in the esters; this separation generally occurs quickly and may be accomplished with either a settling tank or a centrifuge. Water may be added to the reaction mixture after the transesterification is complete to improve the separation of glycerol [13,14]. |
| C. Acid catalyzed Pretreatment |
| Special processes are required if the oil or fat contains significant amounts of FFAs. Used cooking oils typically contain 2-7% FFAs and animal fats contain from 5-30% FFAs. Some very low quality feedstocks, such as trap grease, can approach 100% FFAs. When an alkali catalyst is added to these feedstocks, the free fatty acids react with the catalyst to form soap and water as shown in the reaction below: |
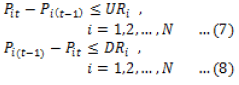 |
 |
| An acid catalyst such as sulphuric acid can be used to esterify the FFAs to methyl esters as shown in the following reaction: |
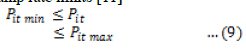 |
| As shown, this process can be used as a pretreatment to convert the FFAs to methyl esters and thereby reduce the FFA level. Then, the low FFA pretreated oil can be transesterified with an alkali catalyst to convert the triglycerides to methyl esters [15]. As shown in the reaction, water is formed and, if it accumulates, it can stop the reaction well before completion. |
| Fatty acids vary in carbon chain length and in the number of unsaturated bonds (double bonds). The structures of common fatty acids are given in Table 1. |
 |
III. USE OF BIODIESEL IN CI ENGINES |
| Biodiesel is a safe, non-toxic, biodegradable and renewable fuel that can be easily used in unmodified diesel engine, and a variety of other applications. The viscosity of waste vegetable oil is very high to be directly used as diesel fuel substitute. The high flash point (>1300C) of biodiesel makes possible its easy storage and transportation. It should be noted that the flash point of petroleum diesel fuel is 640C. |
| Advantages of using vegetable oils as IC Engine fuels are: |
| ïÃâ÷ Produce less smoke and particles, |
| ïÃâ÷ Have higher cetane number, |
| ïÃâ÷ Produce lower carbon monoxide and hydrocarbon emissions, |
| ïÃâ÷ Biodiesels are renewable, biodegradable and non-toxic. |
| ïÃâ÷ Vegetable oils are liquid fuels from renewable sources. |
| ïÃâ÷ They do not pollute the environment with emissions. |
| ïÃâ÷ No modification required to use bio-diesel in CI engines |
| ïÃâ÷ Vegetable oil combustion has cleaner emission spectra. |
| Disadvantages of using vegetable oils as fuels are: |
| ïÃâ÷ Producing high NOx content during exhaust |
| ïÃâ÷ These are not economically feasible yet |
| ïÃâ÷ Need further R&D work for development of on farm processing technology. |
IV. CONCLUSION |
| Alternate fuels for diesel engines have become increasingly important due to decreasing petroleum resource and environmental consequences of exhaust-gases from petroleum-fuelled engines. Biodiesel is an important new alternative transportation fuel. It can be produced from many vegetable oils or animal fat feedstocks. Conventional processing involves an alkalicatalyzed process but this is unsatisfactory for lower cost high free fatty acid content feedstocks. Pre-treatment processes using strong acid catalysts have been shown to provide good conversion yields and high quality final products in case of high FFA feedstocks. These techniques have even been extended to allow biodiesel production from feedstocks like soapstock that are often considered to be waste. However, the high viscosity, low volatility and poor cold flow properties of triglycerides, which result in severe engine deposits, injector choking and piston ring sticking have prevented triglycerides from being used directly in diesel engine. |
References |
|