ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Remya James1, Vineeth Kumar T V2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Amino acid profile of the white and red (dark) meat in Katsuwonus pelamis, a tuna fish, caught from Arabian Sea in the month of January, was estimated using gas chromatography. There was no significant variation between red and white meat samples for the total percentage of essential and non-essential amino acids which constitute 52.3% and 47.7% respectively. Non essential amino acids asparagine and glutamine, and semi essential amino acid cysteine were not detected in both, red meat and white meat samples. Glutamate makes up approximately 13% in both samples which can be considered the highest. Certain individual amino acids, histidine, lysine and arginine showed variation between red and white meat samples.
Keywords |
| red meat; white meat; amino acid; lysine; Katsuwonus pelamis |
INTRODUCTION |
| Fish protein, like that of meat, is easily digestible and favorably complements dietary protein provided by beef, pork, chicken, cereals and legumes that are typically consumed in many developing countries (Winton and Winton, 2000). The protein in fish makes up complete protein source and tuna is a good source of high quality proteins. Tuna have white flesh and flesh that is pink to dark red. The red coloring comes from tuna muscle tissue's greater quantities of myoglobin (Gopakumar, 1997; Bykov, 1983). Proximate composition determined in various tuna fishes (Stansby, 1962; Bykov, 1983; Vlieg and Murray, 1988) shows the importance of tuna in diet. The function of protein in the fish flesh is associated with other components mainly water, ions, lipids and carbohydrates (Damodaran, 1996). The capacity of proteins to modulate adiposity and to enhance immune function and anti-oxidant activity presents new applications potentially suited to the health benefits of the population (Ha and Zemel, 2003). FAO (1985) formulated an „ideal proteinâÃâ¬ÃŸ showing the adequate amount of amino acids. The relative amount of the essential amino acids in an ideal protein is similar to those found in eggs or in cowâÃâ¬ÃŸs milk. Proteins from animals such as foul and fish have adequate proportions of the essential amino acids (Linder, 1985). The digestibility of protein from fish in humans is reported by FAO as 97%. The protein content of most tuna species ranges between 15 – 30% (Bykov, 1983). High quality proteins (proteins having high amount of essential amino acids) such as the protein in fish can be used to maintain an active metabolism and thus can help to lose weight, but low quality protein does not contain all essential amino acids required for use in protein synthesis and means the protein must either be used for energy or converted to fat. The essential amino acid content of fish protein is about 30% higher than that of plant source (Sankar and Ramachandran, 2001). An ideal protein should contain 5.5% lysine, 3.5% sulfur amino acids, 4% threonine, 1% tryptophan and 7% leucine (Krause and Mahan, 1979; FAO, 1985). The chemical composition of fish varies widely between species and among the individual fishes among the same species depending on age, sex, environment and season (Clueas and Ward, 1996). Tuna are considered epipelagic-to-midwater fish, inhabiting the upper and middle layers of ocean water, to a depth of 1,600 feet or more (500 m), depending on size and species. They are found in all oceans, except in polar seas. They occur widely in every tropical ocean. All tunas except the Allothunnus spawn in warm waters (Graham, 1975; Collette et al., 2001). Tuna contains high amount of protein, around 27% and rich in essential aminoacids (Vlieg and Murray, 1988; Gopakumar, 1997; Stansby, 1962; Love, 1970) and thus can be considered as an ideal protein source. This study aims at determining whether both the red and white meat of Katsuwonus pelamis can be given the same status with relation to protein quality. |
II. MATERIALS AND METHODS |
| Analyses of the aminoacids of the red and white meat were conducted as part of quality assessment in the commercial tuna fish, Katsuwonus pelamis (Linnaeus, 1758). The samples were collected in January. Fishes caught from Arabian Sea were collected from the harbor and brought to the laboratory within 10 minutes. The red and white meat were separated, homogenized and was taken for analysis. The total amino acid profile except tryptophan was estimated using Shimadzu HPLC-LC 10As consisting of column packed with a strong acidic exchange resin. The samples were subjected to acid hydrolysis to break the peptide bonds so that the released amino acids can be assayed. Amino acid tryptophan was determined following digestion under alkaline conditions and estimated with thioglycolic acid reagent using UV-VIZ spectrophotometer. The absorbance was measured at 500nm. |
III. RESULTS |
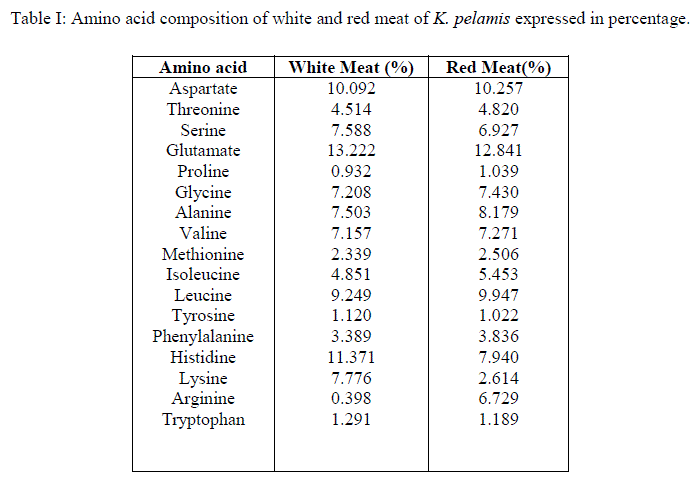 |
 |
IV. DISCUSSION |
| Approximately 52.3% essential aminoacids and 47.7% non essential aminoacids were present in both red and white meat. This means that both red and white meat can be given same status with regard to protein quality. Non essential aminoacids asparagine, cysteine and glutamine were not detected in both red and white meat samples. The sulfur containing aminoacids get partially oxidized during acid hydrolysis (Van de Poll, et al., 2005) and this may the reason for the detection of cysteine in the samples to nill, but methionine was detected in minor percentage, approximately 2.5%. The disappearance of asparagine and glutamine from the amino acid profile can also be attributed to the above said reason, i.e. acid hydrolysis. Glutamine and asparagine get converted to their parent dicarboxylic acids during acid hydrolysis and thus, the peak detected for glutamate and aspartate may be considered as the composite values for their amides. Significant differences between red and white meat samples were found in the percentage of histidine, lysine and arginine. White meat contained approximately 11.4% and red meat contained approximately 8% histidine. Studies by Wolfe et al. (1978) has shown that the white meat of yellowfin tuna contains only 37 to 128 mg% of myoglobin whereas the red meat contains 530 to 22400mg%. There are two histidine groups, a proximal and a distal histidine, attached to a single myoglobin molecule (John et al., 1988; Pamela et al., 2005). Since red meat contains large amount of myoglobin, which render reddish brown color to the meat (Chaijan et al., 2004), a corresponding increase in the amount of histidine was also expected. While 7.8% of lysine was detected in white meat samples, only 2.6% of the same was found in red meat samples. The essential aminoacid lysine along with arginine and ornithine derived from arginine is found to induce somatotropin, mainly in younger individuals by inhibiting somatostatin (Ahmed, 1998). Lysine is considered as the limiting amino acid in the cereal based diet followed by many human populations (Karen et al., 2010). Deficiency of lysine in plant proteins can be thus supplemented by consuming the white meat of tuna and to a less extent, red meat also. This is important because tuna is a commercial fish which is available at moderate price to the middle class people of the society. Arginine, which is attributed to have many health benefits, was detected in minute quantities (approximately 0.4%) in the white meat. Red meat contained approximately 7% of arginine. Arginine is a conditionally non-essential aminoacid (Murray et al., 1991). Therefore consuming red meat along with white meat is necessary to obtain arginine from tuna in diet. Red meat is excluded by most people because of its off flavor and odor (Bertoldi et al., 2004). Formula supplements containing arginine as one of the components has shown to reduce the length of hospital stay in clinical trials which is closely associated with a reduction in acquired infections from the hospital (Bower et al., 1995). Larginine from diet is found to increase efficiency of immune system and inhibit tumours in experimental animals. But in in-vivo studies conducted in humans reveals that it can enhance the progress of tumour grouth (Park et al., 1979). So the study shows that, the subjects who are at the risk of cancer and those who are avoiding tumour promoting biomolecules should restrict the consumption of red meat of tuna, at the same time can safely consume the white meat. Approximately 1% of tryptophan was detected in both white and red meat samples. The B vitamin niacin, the deficiency of which results in pellagra can be obtained from diet as well as the body can make it from its precursor, tryptophan (Paul et al., 2010). Tryptophan supplements are prescribed for sleeplessness, depression, to relieve pain, to regulate appetite, mood and sensory perception. But excess intake of these supplements has many harmful effects (Frances et al., 2011). Consumption of food containing enough tryptophan can be recommended as a safe source instead of supplements and the present study shows that both the red meat and white meat can provide tryptophan. The recommended daily allowance (RDA) for tryptophan is 500mg/day (Food and Nutrition Board, 1968). An ideal protein should contain 5.5% lysine, 3.5% sulfur amino acids, 4% threonine, 1% tryptophan and 7% leucine (Krause and Mahan, 1979; FAO, 1985). The results show that both red and white meat samples of Katsuwonus pelamis satisfy the ideal protein features except for sulphur aminoacids which is found to be 2.5% and lysine in red meat (2.6%). |
V. CONCLUSIONS AND RECOMMENDATIONS |
| The amount of individual amino acids present in red and white meat of Tuna fish Katsuwonus pelamis varies significantly. Both red and white meat can provide required essential amino acids and thus can be considered equally important in human diet. |
References |
|