ISSN:2321-6212
ISSN:2321-6212
Department of Chemistry, College of Science, Shahid Chamran University, Ahvaz, 65355-141, Iran.
Received: 16/10/2013 Accepted: 12/12/2013
Visit for more related articles at Research & Reviews: Journal of Material Sciences
NiO nanoparticles have been prepared by a simple and efficient chemical precipitation method with the aid of a surfactant. In this method, NiO2 (nickel peroxide) was first prepared by oxidation of nickel (II) nitrate with hypochlorite solution. The as-prepared NiO2 was then easily converted to nanosized NiO merely by treating it with ethanol at room temperature. The fabricated NiO nanoparticles were characterized by XRD, TEM, and EDX techniques. The effect of three different surfactants viz., SLES, CTAB and Triton X-100 on the formation of NiO nanoparticles was also studied
Nickel oxide, Nanoparticles, Surfactant, SLES, CTAB, Triton X-100.
In recent years, nanostructured materials have received steadily growing attention as a result of their peculiar and fascinating properties and applications superior to their bulk counterparts [1,2]. Among the various nanomaterials, metal oxides have attracted increasing technological and industrial interest. This interest has mainly to do with their properties (optical, magnetic, electrical, and catalytic properties) associated with general characteristics such as mechanical hardness, thermal stability or chemical passivity [3-5].
Transition metal oxides can be prepared through various methods, such as ultrasonic spray pyrolysis [6], liquid-control-precipitation [7], electrodeposition [8], chemical vapor deposition [9], the sol–gel route [10], reduction of metallic salts followed by oxidation of metallic species [11] and pulsed laser ablation [12].
The characteristic properties of NiO nanoparticles enable one to fabricate and tailor these nanoparticles for a variety of applications including catalysis, electrochromic windows, battery cathodes and sensors [13-18]. So far, many synthesis methods have been developed for the preparation of NiO nanoparticles [19-22]. The chemical methods, including the precipitation of Ni2+ ions with NaOH, NH4HCO3, CO(NH2)2 or NH3 from an aqueous solution are more promising routes because of inexpensive raw materials and simple processing [23-26]. However, in most of all these methods, calcinations at the minimum temperature of 250°C are needed to get the crystalline NiO. In fact, the precipitation preparation of NiO nanoparticles is composed of two stages: the formation of meta-stable nickel precursor precipitate and the subsequent transformation of this precipitate to nano-NiO by thermal treatment [7,26]. Therefore, various parameters like pH, temperature and solution concentration have to be controlled because they play significant roles in the preparation of nanoscaled NiO. If, for instance, the meta-stable nickel precursor is to be prepared by addition of ammonia to Ni2+, a reaction according to Equation 1 takes place.
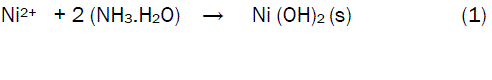
In this case, the concentration of ammonia has to be carefully controlled because any excesses of this reagent will dissolve the precipitate according to Equation (2).
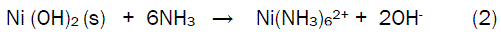
Therefore, these precipitation methods have their own complications and the development of a facile preparation process that allows convenient production of NiO nanoparticles is necessary for practical application. In this study, nano-crystalline NiO powder is prepared via a simple and rapid procedure. The method is based on oxidizing of Ni2+ to NiO2 (nickel peroxide) and then converting this peroxide to NiO by treating it with ethanol in the presence of a surfactant. The use of surfactant is in fact somehow similar to the reverse - micellar method which has been recently demonstrated as a versatile route to produce a variety of nanoparticles [27-30]. The effect of different surfactants on the size of the as-prepared nanosized NiO is also investigated. The present method is superior to the previously reported methods of preparing NiO nanoparticles [31,32].
All chemicals such as Ni (NO3)2·6H2O (nickel nitrate hexahydrate), were of analytical grade, and they were used without further purification. Deionized water was used throughout the study.
Nickel oxide nanoparticles were prepared using the following process: A solution containing 0.02 mol of Ni (NO3)2·6H2O in 15 mL of deionized water and 5 g of either of the surfactants, cetyltrimethylammonium bromide (CTAB), sodium laurethsulfate (SLES) or Triton X-100 was prepared and this mixture was magnetically stirred for 15 min. This solution was then treated with a mixture of 15 mL of sodium hypochlorite solution (bleach, 6% solution) containing 0.04 mol of NaOH under continuous agitation. The black precipitate of NiO2 was formed in this stage. The obtained NiO2 was filtered off, washed with deionized water and then put in a beaker containing 20 mL of ethanol. On stirring of this suspension for 2 h, the black solid turned to olive green indicating the formation of NiO. After the reaction was complete, the solid powder was filtered off, washed with deionized water several times and then dried in an oven at 90°C for 5 h to afford nanosized NiO (95% yield).
The as-prepared NiO nanoparticles have been characterized by X-ray diffraction (XRD), transmission electron microscopy (TEM) and energy dispersive spectroscopy (EDS). The XRD patterns were obtained on a Philips Analytical X-ray diffractometer, operating with CuK α radiation (λ=1.54056 Å), with a flat sample holder mounted on a PW1830 spectrogonimeter. The EDS measurements were carried out on a LEO 1455 VP energy dispersive spectrometer. The TEM images were taken using a LEO 906 E transmission electron microscope.
In our procedure of NiO nanoparticcles fabrication, we have first synthesized NiO2 through oxidation of nickel nitrate with an alkaline sodium hypochlorite solution as per a reported procedure [33]. The preparation of NiO2 was carried out in the presence of either, CTAB, SLES or Triton X-100 as real micelle-forming agents. The black NiO2 powder was readily converted to NiO, as olive green solid, merely by stirring it in ethanol at room temperature.
The XRD patterns of the synthesized NiO nanoparticles are shown in Fig.1. All these diffraction peaks can be perfectly indexed to the face-centered cubic (FCC) crystalline structure of NiO, not only in peak position, but also in their relative intensity of the characteristic peaks, which is in accordance with that of the standard spectrum (JCPDS, No. 04-0835). The XRD pattern shows that the samples are single phase and no any other impurities distinct diffraction peak except the characteristic peaks of FCC phase NiO was detected. This result shows that the physical phases of the NiO nanoparticles prepared in this work have higher purity. It could be seen that the XRD patterns for the three NiO samples prepared by using different surfactants are the same except for varying intensity and broadness of the peaks. The broaden diffraction peaks indicate that the samples are on the nanometer scale. The average grain size (D) from X-ray line broadening has been calculated using the Debey–Scherrer equation [34]. In this equation which has the following form,
D = K λ / β cosθ
D is the mean diameter of the grains, K is the so-called shape factor, which usually takes a value of about 0.9, λ is the wavelength of the X-ray source used in the XRD, β is the full width at half maximum and θ the diffraction angle of the peak. The mean particle sizes of the as - synthesized NiO samples estimated from Scherrer equation are 20 nm, 35 nm, and 40 nm in the presence of the surfactants CTAB, SLES and Triton X-100 respectively.
TEM images of the as-prepared NiO nanoparticle samples are shown in Fig.2. It can be seen from Fig. 2 that the NiO particles have nearly spherical shapes and are well dispersed, with weak agglomeration. The mean sizes of NiO particles are 12 nm, 16 nm and 24 nm for the surfactants CTAB, SLES and Triton X-100 respectively. The average sizes of NiO nanoparticles calculated by Scherrer formula are somewhat larger than the values observed with TEM analysis. However, both these methods of grain size measurements clearly indicated that the cationic surfactant, CTAB, produced the smallest grains sizes of NiO particles. On the other hand, the nonionic surfactant, Triton X-100, gave the largest sizes of NiO nanoparticles. The average nanoparticles sizes obtained with the XRD and TEM methods are given in Table 1.
Presumably, the ionic surfactants in solution can surround the nanoparticles via their positive or negative ends to prevent these particles from being agglomerated and therefore decreasing the particle size of the nanoparticles. The use of surfactants as micelle-forming agents, such as PEG-400, has also been reported for the synthesis of other metal oxides [35,36].The present method, however, is superior to other synthetic methods of a kind as it produces homogeneous, uniform and mono-disperse nanosized particles. In addition, the preparation method is an easy one and there was no need for heat treatment.
The as-fabricated NiO nanoparticles were further characterized by EDS elemental analysis. As shown in Fig. 3, the EDS spectrum of one NiO sample (one which prepared in the presence of CTAB surfactant) indicates the existence of only Ni and O elements. The other nanoparticles NiO samples obtained in the presence of SLES or Triton X- 100 surfactants show the same EDS spectra as the one shown in Fig. 3. The obtained results of the EDS elemental analysis are given in Table 2. As it can be seen from the information in Table 2, the experimental percentage of Ni and O elements match closely with the theoretical amounts of these elements.
We have shown that NiO nanoparticles can be readily produced by a rapid and efficient method. This method is based on oxidation of Ni2+ to NiO2 first and transforming of the latter to NiO by treating with ethanol in the presence of a surfactant at room temperature. The as-prepared NiO nanoparticles are obtained with high yield and purity. In our procedure there is no need for controlling the pH of the solution, as it usually required in most of the chemical precipitation methods. More importantly, calcinations at high temperature, which is used in other similar routes, have been eliminated in the present method. Therefore, this method can be introduced as an inexpensive, fast and reproducible process for the large-scale synthesis of NiO nanocrystals. The use of cationic surfactant CTAB which produced smaller nanosized NiO is recommended for this purpose.
The authors are grateful to the Research Council of Shahid Chamran University, Ahvaz, Iran, for the financial support of this research.