ISSN:2321-6212
ISSN:2321-6212
College of Pharmaceutical Sciences, Govt. Medical College, Thiruvananthapuram – 695011, Kerala, India.
Received: 21/06/2013 Revised: 03/07/2013 Accepted: 11/08/2013
Visit for more related articles at Research & Reviews: Journal of Material Sciences
Direct compression is the preferred method for tablet manufacturing due to the simplicity in processing and its cost effectiveness. However, for applying direct compression in tablet manufacturing, the drug used should have good flow properties and compaction characteristics. Many drugs are lacking these properties and so it is not possible to compress them directly into tablets. Crystallo co agglomeration (CCA) is an innovative technique developed with intends to provide the drugs with good micromeritic and mechanical characteristics. The process of CCA involves crystallisation followed by simultaneous agglomeration of the drug with the aid of a good solvent and /or a bridging liquid and a bad solvent. It is also possible to incorporate other drugs (to get a combination tablet), excipients (e.g. disintegrants for fast dissolving tablets) and different polymer combinations (to modify the drug release properties). In the recent years,attempts were made to produce Crystallo co agglomerates of various drugs, whichwere briefly discussed in this article. Even though large scale applications of CCA is not yet made possible, this technique gives a new line of opportunities to the tablet manufacturing process, ensuring low cost, single stepped production of particles with good micromeritic and mechanical characters which can be directly compressed
Crystallo co agglomeration, good solvent, bridging liquid, direct compression, Micromeritics.
Direct compression method is the most preferred method in the manufacturing of tablets, if the choice of excipients is appropriatebecause of its simplicity, economy and potential for high volume output [1,2].Batch sizes of tablets manufacturedby direct compression is affected by the capacity of the equipment used. Due to the absence of heat and water, the stability of tablets formed by this method is better. The tablets produced by this method have good dissolution as well as faster disintegration [3].
However, there are certain disadvantages, which make the practical application of it to a limited range of products. It includes problems with relative density and flow properties. In order to overcome the above difficulties the particles should have larger particle size. As a result, direct compression was done mainly to two classes of compounds.
• Drugs containing coarser particles.
• Highly potent drugs to with which large amount of coarse excipients could be incorporated.
Kwashimaet aldeveloped a new technique ‘Spherical crystallisation’ to overcome these problems. This technique also indents to modify the crystalline nature of drugs. Nevertheless, its application was restricted to size enlargement [4].Crystallo co agglomeration is an extension of spherical crystallisation and was developed by Kadamet al in 1997 [5].
Crystallo co agglomeration or CCA is a novel technique in which drug or drugs or excipients are crystallised and agglomerated simultaneously from a good solvent and or bridging liquid by the addition of a non-solvent [6]. This single step process was done with one, two or more drugs -small dose or large dose with or without diluents. The spherical agglomerates thus generated made into intact beads (encapsulated spensules), intermediate of directly compressible tablets with improved micromeritic (flowability), mechanical (friability), crushing, compressional (compressibility, compactability) properties. The drug release properties were controlled by the selection of proper polymer composition. The main features of CCA are its simplicity owing to the single step operation and lower production cost.
The spherical agglomerates produced by this technique have been used in the design and manufacture of Multiple Unit Particulate Drug Delivery System [7]. Blank talc agglomerates were used as an inert core or coating substrate by the aid of which drug loaded talc pellets can be prepared. This drug loaded talc pellets have better mechanical, micromeritical and compressional properties [7-9]. With the selection and use of appropriate polymers, it is possible to compress into tablet intermediate and or spensules, which possess all advantages, required in the design of MUPS.
• Excellent flow properties.
• Uniform size distribution.
• The process is very simple
• Less processing cost which makes the production economic.
• Unit operations are minimal.
• Single step generation of agglomerates.
• The process requires less labor - one personal required for entire operation.
• The simplicity in the process helps in enabling the manufacturer to comply easily with CGMP.
• Crystallo co agglomerates can be used as tablet intermediates and for the design of MUPS.
• Large surface area that enables uniform distribution of drug through gastro-intestinal tract. This in turn helps in reduced toxicity, improved absorption and thus adequate bioavailability.
• The low surface area to volume ratio makes them excellent waiting substrates
• They have good therapeutic qualities due to improved dosing and handling properties.
• They are least affected by gastric emptying.
• Their drug delivery is less prone to physiological variables.
• They show less dose dumping.
• If the pellet size is less than 2.4 mm diameter, then they are free from gastric digestive function and closing system of pyloric sphincter.
• Use of organic solvents is un-avoidable. Relatively non-toxic solvents should be used.
• Drug combinations having similar physicochemical properties are difficult to process in crystallo co agglomeration.
• If the external phase volume is more, it will result in the increased loss of drugs and the resistance for mixing of contents also increases. The increased resistance will increase the power requirement.
• It is difficult to incorporate disintegrants or super disintegrants since the external phase is aqueous.
• The process involves multiple functional variables and process variables, which causes difficulty in reproducing the same results.
• It is difficult to scale up the filtration and drying steps.
The important factor in the process design of crystallo co agglomeration is the solvent system [10]. The solvent used contains three components- a good solvent (volatile), bridging liquid and a non-solvent. The good solvent solubilises the drug while the non-solvent causes the crystallisation or precipitation. It is essential that the bridging liquid and good solvent must be immiscible. The bridging liquid helps in the formation of crystal bridges between crystals and the insoluble particles during the process of agglomeration [11]. In times, they act as good solvent also [12-13].
As most of the drugs are non-polar and highly soluble in organic solvents, they are preferred as the good solvent as well as the bridging liquid. So automatically, an aqueous solvent is used as the non-solvent.
The process of Crystallo co agglomeration can be done in two ways [14]. Solvent change is the most commonly used method for obtaining crystallo co agglomerates. The two methods are discussed below:
In solvent change method, Crystallo co agglomerates can be obtained by the crystallisation as well as the agglomeration. It takes place to one or more drugs simultaneously from the system containing good solvent and bridging liquid by the addition of a non-solvent.
In the alternate method,first, the crystallisation of the drug is done from a system containing good solvent and bridging liquid and then its simultaneous agglomeration is carried out with an insoluble diluent or a drug by the addition of a non-solvent.
Either of the above-mentioned methods can be chosen for carrying out Crystallo co agglomeration, but the selection is based on the physico chemical properties of the drug.
The processing was done in a vessel designed and developed by Morishimaet al for spherical crystallisation. It have a motor type propeller, a baffle which are enclosed in a vessel which is lidded and having provision for insertion of various ingredients. The vessel is placed in a thermostatically controlled water bath.
Controlled agitation is required for the proper formation of agglomerates.
The end point of the process can be determined by the clarity of the supernatant and vaporization of organic solvent from the agglomeration system.
The process was done in Morishima vessels, which werementioned above. Successful crystallo co agglomeration can be ensured by good crystallisation and agglomeration.
The homogeneous mixture of polymers, diluents, drugs, surfactants, disintegrants etc. are placed in the vessel [16]. The above mixture is wetted by the addition of good solvent and the bridging liquid. Constant stirring is done to ensure the formation of a homogenous mass. The walled baffles are placed in the vessel. The aqueous solution of polymers in non-solvent is then introduced into the vessel. The process is carried out with a continuous stirring at a constant speed for a definite time period until the Crystallo co agglomerates are formed. The end point of the process depends on the size of agglomerates, clarity of supernatant and vaporisation of the solvent from the system. After the completion of process the whole mass is filtered or decanted and washed. Wash thoroughly so that the complete removal of solvents can be ensured since organic solvents are more or less toxic. For washing, the filtrate itself is used since it prevents loss of drugs and polymers from the Crystallo co agglomerates. The obtained agglomerates are then dried in a hot air oven for 24 hours.
These are used for the enlargement of size in low dose drugs. The main characters required for diluents in this process are
• They should be physico chemically and physically inert and inexpensive.
• They should be insoluble in aqueous phase so that the drug loss through the continuous or external phase can be avoided.
One of the most commonly used diluents is talc [17]. Linzerwala was the first scientist who formulated placebo beads using talc. Later Gadika and Judah [18] formulated bromhexine hydrochloride using talc for size enlargement. Pawaret aldeveloped high dose ibuprofen agglomerates using talk. Oral use of talc is not restricted since it does not produce any gastro intestinal disorder.
The Temperature has an effect in the size, shape and strength of the agglomerate. Various studies indicate that the increased temperature will result in increased solubility and as a result there will be more drug loss through supernatant liquid. In most of thecases, temperature is kept as low as 50c [19].
It was found that the Crystallo co agglomerates pure drugs have poor compressibility and handling qualities. This will prevent the use of direct compressing in tablet making and thus fails the purpose. So various polymers like hydroxy propyl methylcellulose (HPMC), poly ethylene glycol (PEG), ethyl cellulose (EC) and poly vinyl pyruvate (PVP) were used.This improves the micromeritic mechanical and drug release properties of the agglomerates.
Hydroxy propyl methyl cellulose
• Provides adequate sphericity and mechanical strength to the agglomerates when used in optimum quantity.
• The excess addition of HPMC will result in deformation and ellipticity of the agglomerates.
Poly ethylene glycol
• It will reduce the interfacial tension between external phase (water and bridging liquid). Thus it reduces the cohesive force and produces small sized agglomerates.
• The agglomerates formed using PEGs are soft and plastic in nature. This will cause plastic deformation and there by provides better compressibility.
Ethyl cellulose
• It provides high yield strength (On crystallisation in non-solvent imparts more strength to the agglomerates)
So in conclusion to have agglomerates of satisfactory sphericity, strength and compacts having adequate strength the combination of HPMC, PEG and EC in appropriate proportions are used.
The internal phase (drug suspension with or without diluents and bridging liquid) should be easily dispersed or emulsified in the external phase. This step can be assisted by distributing agents or dispersants. For this purpose surfactants and hydrophilic polymers like polysorbates, PVP, Polyvinyl, alcohol (PVA) can be used in optimum concentrations.
It was found that the extent of drug loading changes the requirement of solvent system. It has a pronounced effect on the quality of agglomerates. Increased drug loading may result in increased loss of drug through external phase. If the system has an insoluble diluents or excipient there is a chance for the crystallised drug to get deposited on its surface.
The loss of drug through supernatant liquid has a significant role in determining the extent of drug entrapment and the overall efficiency of the crystallo co agglomeration. It should be ensured that maximum crystallisation and agglomeration occurs during agitation. In order to minimize the loss of drug to the supernatant fluid the following points should be assured.
• Maintaining low temperature.
• pH adjustments.
• Addition of solubility suppressants to the external phase.
The process yield depends on the amount of crystallisation occurred from the good solvent as well asthe extend of agglomeration from the bridging liquid. Thus the selection of solvent system holds an important role in the process yield of crystallo co agglomeration. The solubilisation of drug is determined by the good solvent and the crystallisation is done by the non solvent. The bridging is an interparticular interaction. Hence for obtaining desirable yield proper selection of solvent system is recommended.
The main function of agitation is emulsification or dispersion. The size, shape, sphericity and strength of the agglomerates were affected by agitation. High speed agitation may result in increased sphericity and decreased strength of the agglomerates. It was also found that with the increase in speed of agitation, it may decrease the time required for the process and itdecreases the agglomeration.
The time of agitation decides the completion of agglomeration. Incomplete agitation leads to incomplete mixing of various ingredients, thus incomplete growth of agglomerates. This also reduces the evaporation of organic solvents from the reaction vessel, while excess agitation result in fine formation [21].
The end point of agglomeration determination is critical in CCA. It can be found out by judging the clarity of the supernatant, residual organic solvent and attainment of proper agglomerate size.
In surface topography studies,the agglomerates were photographed using an optical microscope with camera at its original magnification. The area (A) and perimeter (P) of the agglomerates were obtained from tracings of enlarged photomicrographs. This can be used to calculate shape factor (S).
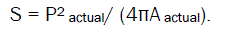
Twenty granules per batch can be evaluated.
Differential scanning calorimetry includes the measurement of changes that occur when heat flow to the sample while they are subjected tocontrolled temperature programming.
DSC studies the thermotropic behaviour of particles. The process like crystallisation can be observed using DSC. When temperature of a sample is increased gradually the viscosity of amorphous solids will decrease. At a particular point the molecules may attain sufficient energy so as to arrange themselves into crystals. This temperature is crystallisation temperature (Tc). This process of conversion of an amorphous solid into a crystalline solid is an exothermic process and is indicated in the thermogram (graph obtained) as a peak. This principle is used in the analysis of crystallo co agglomerates.
Thermograms of drugs, polymers and agglomerates are performed using a differential scanning calorimetry. The DSC temperature should be calibrated. Accurately weighed samples are sealed in an aluminium crucible. The system should be purged with nitrogen gas.
It is a technique used for the structural characterisation of powders with the aid of X- ray, neutron etc. The basic principle behind this is that in a powder sample every possible crystalline orientation can be equally represented. The powder diffraction data is represented in the form of diffractogram. In diffractogram, the diffracted intensity is shown as a function of scattering vector or scattering angle. The instrument used in the study of X-ray diffraction of powders is known as powder diffractometer.
Powder X ray diffraction patterns of the drugs and agglomerates should be taken and evaluated.
Agglomerates should be evaluatedformicromeritic properties to get proper data for future studies and to ensure quality.
It can be studied by sieve analysis.
By using Rosin-Rammlerdistribution [24]
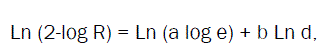
Where,
R- Cumulative residual percentage by weight,
d- The particle size (μm),
a and b-constants.
They are measurements of propensity of a powder to be compressed. The Carr’s index is considered as an indirect measure of bulk density, size and shape, surface area, moisture content and cohesiveness of materials since all these can influence the Carr’s compressibility index. The determination of both Carr’s index and Hausner’s ratio include the measurement of bulk volume and tapped volume of the powder.
Carr’s index = 100 (Vo-Vf) / (Vo)
Hausner’s ratio = Vo / Vf
Where,
Vo –Unsettled apparent volume
Vf – Final tapped volume
It is defined as the maximum angle possible between the surface of a pile of the powder and the horizontal plane. It is given by the equation
Tanθ = h / r
Where,
h – Height of the pile
r – Radius of the base of the pile
θ – Angle of repose
Crushing strength of agglomerates is of 3 different size fractions (i.e. 855, 567, and 390μm) can be determined by using mercury load cell method [26]. Friability of agglomerates can be tested by subjecting them to attrition. After sieve analysis, every time mean geometric diameter should be obtained fitting the data in Rosin-Rammler distribution. The Percentage friability index (FI) can be calculated using the following equation,
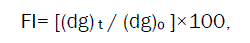
Where,
(dg) t and (dg) o- Mean geometric diameters after time t and initial time, respectively.
Agglomerates (say 500±10 mg) can be compressed at compaction pressures of 0.52, 1.57, 3.15, 4.20, 5.25, 6.30, and 14.70 mPa for 1 min using a hydraulic press. The compacts should be allowed to relax for 24 h.
The Pressure (P)- relative density (ρr ) data were analyzed using the Heckel Equation
Where,
K - Heckel constant
K= 1/3σ0
Where,
σ0 - Yield strength
PY - Mean yield pressure equal to 3σ0.
A- Constant, it express the densification at low pressure.
After determination of diameter (D) and thickness (t), the compacts used for P-ρrrelationshipcan be used to determine the force (F) which is required to break the compacts. The data obtained can be subjected to tensile (σt ) determination,
σt= 2F/π Dt.
The agglomerates formed by CCA is then subjected to dissolution studies in order to understand the pharmacokinetics and thereby bioavailability of the obtained product. U S P recommends seven apparatus for the dissolution studies, which include rotating basket type apparatus, rotating paddle apparatus, reciprocating cylinder apparatus, flow through cell apparatus, paddle over cell apparatus, cylinder apparatus, and reciprocating disc apparatus. Any of the above devices can be used for dissolution studies depending upon the type of tablet evaluated i.e. whether conventional, controlled release etc. on the basis of dissolution profile data,criteria for acceptance or passing of test results are given in the following table.
The agglomerates obtained should be strong, non-brittle,should have low elastic resistance, should deform under pressure without forming fracture. However, experimental studies reveal that better compressibility of the powders is due the fracture formation [29].
• Drug layering.
• Agglomerates produced using either starch or talc act as matrix beads.
• CCA is useful in making agglomerates of one, two, or more drugs, high dose or low dose, with or without excipient.
• It is possible to directly compress those drugs, which were unless otherwise impossible to do so, with improved micromeritic, mechanical, compressibility, compactability properties possessed through this technique.
• Drug uniformity of agglomerates is unique.
• It is possible to make controlled release dosage forms with the proper selection of polymers.
• Improved dissolution characteristics and bioavailability.
• It is possible to create placebo drugs by producing agglomerates of plain excipients (talc agglomerates )
• It is possible to formulate agglomerates in the form of encapsulated dosage form as MUPS.
• The shear required for compression is less than that of granules.
• The entire process can be controlled by a single person.
• The requirement of time and space are less.
• As the process contains only single step it is possible to carry out it in a closed system. This prevents external contamination. So it is easy to follow cGMP.
Crystallo co agglomeration: A novel technique to obtain ibuprofen- paracetamol agglomerates was published in a journal [19]. Ibuprofen – paracetamol agglomerates are made using DCM- Water as solvent system. The agglomerates obtained contain desirable drug ratio. The DCM act as both as a good solvent and bridging liquid. Water is the non solvent. The polymers used are PEG 6000 and PVP ethyl cellulose. pH is maintained at 5 owing to the solubility of Ibuprofen and stability of paracetamol. The loss of paracetamol to the supernatant can be minimized by keeping temperature low and by the addition of paracetamol as the solubility suppressant. More over the increased temperature resulted in interaction of the individual components. The purpose of ethyl cellulose is to provide mechanical stability. PEG is used to improve the compression properties of the particles. It also affected the drug release at initial stages. It is also found that the nature & properties of the agglomerates and the agglomeration are influenced by the polymer nature.
There was a research paper on the agglomerates formed by ibuprofen and talc [32]. The percentageprocess yield is of 95-96% w/w. DCM – Water system is used as the solvent system, where DCM act as good solvent and bridging liquid as in the previous case. They found that the form of ibuprofen used and the hydrophilicity of the talc affect the drug release. At higher proportion of talc, the drug release is found to be zero order. In some proportions the drug release were extended to about 13 hrs.
A work is done regarding the effect of various concentrations of polymers like ethyl cellulose, hydroxyl propelmethylcellulose and poly ethylene glycol on the agglomerates formed. Agglomerates obtained are of Paracetamol and ibuprofen which is prepared by recrystallization of ibuprofen followed by simultaneous agglomeration of paracetamol. DCM is employed as the good solvent as well as the bridging liquid, whereas an aqueous phase with dextrose dispersed in it is employed as the bad solvent. The temperature of the process is kept below 5 c to minimize the loss of the drug to the supernatant.
When HPMC is used as the polymer, it will increase the agglomeration size,compactability between drugs in the agglomerates and will increase the resistance of agglomerates to abrasion.However, an increase in the concentration of PEG tends to decrease the amount of paracetamol present in the agglomerates. Surface topographic studies show spherical agglomerates. But increased concentration of PEG caused deformation on the surface. A combination of PEG and EC at appropriate concentration gave the agglomerates an increased resistance against fragmentation and also an improved elasticity modulus.
The effect of PEG 4000 on the size of the agglomerates using phenytoin as drug was also studied [30].Isopropyl acetate acts as the bridging liquid. The average diameter of the agglomerates is determined. It is found that at equilibrium that is the point where the rate of agglomeration equals the rate of deagglomeration, crystal formation decreases with increase in concentration of PEG. PEG decreases the cohesive force tending to agglomerate the crystals to the bridging liquid by decreasing the interfacial tension and wettability of the liquid.
Recently a research work was published based on the crystallo co agglomeration of olmesartanmedoxodil.The problem with the drug in direct compressing arises due to its low aqueous solubility and resulting decline in the micromeritic and dissolution properties. For thesestudies, thermodynamically stable crystals were preferred. Inadequate stability and dissolution will result in less oral absorption and bioavailability. DCM is used as a bridging liquid. The polymers used were PVP and hydroxypropel β cyclodextrin [32].
The particle design of Aceclofenac- disintegrant agglomerates for direct compression by crystallo co agglomeration technique was also a research work done. The solvent system includes acetone, DCM and water system of which they act as good solvent binding liquid and non-solvent respectively. PEG 6000, HPC, starch glycolate(SSG), cross povidone (CP) and croscarmellose sodium (CS) are the polymers used in whichtheincorporation of 18.43% CS shows shortest disintegration time(18.3s) and maximum drug release. PEG is used in aconcentration of 6.5% w/w of the total solid content and HPC used in the concentration of 10% w/w of drug and disintegrant. Aceclofenac is an anti-inflammatory agent used in osteoarthritis conditions like rheumatoid arthritis and inflammatory diseases of joints [33].
Agglomerate formation of naproxen was also studied using acetone (good solvent), water (non-solvent), and hydroxypropel cellulose (bridging liquid) [34]. The conversion to agglomerates increasesthe size of individual particles and these particles possess good spherical shape and flow properties also improved considerably. It is also found that the improved compactability of the agglomerates were due to the fragmentation of the particles during the process. The naproxen drug molecule did not undervent any structural changes. By varying the amount of disintegrant used,the dissolution rate can be controlled. The main advantage of producing naproxen disintegrant agglomerates were that the better dissolution of drug when compared to naproxen drug crystals and that using this technique there is no need for physical blending of disintegrants.
Bromhexine hydrochloride talc agglomerates can be prepared using DCM as the good solvent and the binding liquid whereas water serves as the bad solvent. Talc acts as the diluent. To aid the dispersion of bromohexine hydrochloride Tween 80 is used as the dispersion agent. The polymers used are HPMC (50cps) 4%w/w for providing mechanical strength to the agglomerates, PEG 6000 5% providing adequate sphericity to the agglomerates. In the optimum batches the agglomeration yield and the drug entrapment percentage is found to be above 94%. By adjusting the amount of polymers and diluents it’s possible to attain sustained release preparations of release ranging from 9-5 hrs. The angle of repose obtained was 29- 30 indicating good flow properties. Compression studies indicated that the deformation of the particles occurs rather than fracture. Low porosity also accounts for this.
Nimesulide a non-steroidal anti-inflammatory drug used in arthritis can also be made into agglomerate [35]. The drug also causes a retarding effect in Alzheimer’s disease.Thenimesulide at its conventional form had very poor flow properties. By this technique the flow property of nimesulide can be enhanced. The half-life of the generated agglomerates was found to be 2-5 hrs. In this study DCM is used as both good solvent and bridging liquid. The drug release rate is controlled by using PEG & EC. The agglomerates obtained also had higher yield strength and greater degree of plasticity. The analytical data of the particles are given inthe following table.
The crystallo co agglomeration of secnidazole an antimicrobial agent is carried out using acetone petroleum ether system[36]. The aim of this study is to learn the influence of polymers and excipients on developed particles.The spherical shape of the obtained particles ensured excellent flow, packability and compactability while better crushing strength ensures good handling characteristics. The lower σ value in the Heckel plot, higher tensile strength and lower elastic recovery ensured excellent compressibility. They also had improved dissolution. The research concludes that CCA is an excellent alternative to the wet granulation process to prepare the particles for direct compression. The use of polymers and other excipients improved the processability.
Ketoprofen talc Crystallo co agglomerates was also prepared in DCM-water solvent system [37]. The polymers used were PEG 6000, PVA and HPMC 100 centipoise. The particles obtained shows better flowability. By the use of lower amount of polymers sustained release products are obtained. The kavakita constant is small indicating higher packability. Increased ‘k’ value in kunos equation indicates greater rate of packing.
Aceclofenac paracetamol agglomerates are prepared using acetone (good and bridging solvent) and water (bad solvent) [38].Polymers like PEG 6000, PVA, HPMC etc are used to enhance various properties of the agglomerates. The agglomerates obtained were of smooth surface and spherical shape. The higher the amount of HPMC and PEG used the higher the surface strength of the agglomerates formed.
Meloxicam is an NSAID which has numerous difficulties in formulation including poor solubility, poor flow properties, difficulty in direct compression etc. To overcome these many shortcomings meloxicam agglomerates can be prepared [39]. The system used for the preparation of agglomerates include an aqueous system with water, one third of disintegrant, polymers like HPMC, PEG 6000 etc. The drug is dispersed in good solvent (acetone) with two third of disintegrant present in it.
Table 4 shows the works held on CCA till this date and Table 5 shows agglomerated drugs and their therapeutic activity [39,40].
DCM-dichloromethane; PEG-polyethylene glycol; PVP-poly vinyl pyrrolidine; EC-ethyl cellulose; HPMC- hydroxypropel methyl cellulose; SSG-sodium starch glycolate; CP-crospovidone; CS- croscarmellose.
Crystallo co agglomeration is a novel technique used for the size enlargement process in the micromeritic mechanical and drug release properties of drug which in the conventional form cannot be directly compressed. It is a cost efficient single step process. The main drawback of the process is its inability to scale up the process and the use of organic solvents (toxicity). The agglomerates generated can be used as direct compression and spensules in MUPS.
Various studies conducted shows very good flow properties indicated by Hausner’s ratio (near 1), angle of repose (20 – 30). The compressibility also improved while using the Crystallo co agglomerates for direct compression compared to the parent particle. It will be a revolutionary change in the tablet manufacturing industry if Crystallo co agglomeration can be up scaled. It will lead to very low cost tablets, whose batch size is only limited by the vessel size, a manufacturing process which only has a few steps, thus following cGMP standards will be very easy. The number of technical people in the manufacturing unit can also be reduced. So it can be certainly considered as a revolutionary step in pharmaceutical