e-ISSN: 2320-0812
e-ISSN: 2320-0812
Om Prakash*, S Saraf, M Rahman, Neeraj Agnihotri, and Vinay Pathak
Department of Industrial Chemistry, Integral University, Lucknow, Uttar Pradesh, India
Received date: 25/08/2013; Revised date: 06/09/2013; Accepted date: 28/09/2013
Visit for more related articles at Research & Reviews: Journal of Pharmaceutical Analysis
Nateglinide is a novel, highly physiologic, glucose regulator recently approved for the treatment of type-2 diabetes mellitus. Nateglinide has a rapid onset and short duration of insulinotropic action that results in reduction of glucose level. The purpose of this study to develop a floating tablet of Nateglinide to enhance its bioavailability and sustained action. In the present study a 3² full factorial design was employed in which 2 factors were evaluated at 3 levels, experimental trials were performed at all possible 9 combinations. The independent variables selected for this study were concentration of Ethyl cellulose (X1) and sodium bicarbonate (X2). % drug release for 30min,1h, 2h,4h,6h, 8h,12h, 16h,24h and floating lag time (FLT) were as dependent variables. The results of factorial design showed that factor X1 and X2 significantly affect the studied dependent variables. The floating tablet formulations were evaluated for Bulk density (gm/cm3), Tapped density(gm/cm3), Hausner ratio(HR), Carr index, Angle of repose, flow property, assay, in-vitro drug release, hardness, friability,weight variation. The results of in vitro release studies showed that the optimized formulation (F8) could sustain drug release (98.33%) for 24h. Stability of tablets at 400C/75%RH, of optimized formulation was carried for one month and no significant change was observed.
Nateglinide, 32 full factorial design, floating tablet, Ethyl cellulose, sodium bicarbonate, sustain drug release.
Oral delivery of drugs is most preferred, versatile and convenient route of drug administration because of ease to use, economical and safe. Drug bioavailability of Oral controlled drug delivery is influenced by various factors [1] Gastrointestinal(GI) physiology [2] Physiochemical properties of drug [3]. Dosage form charectristics [4]. Patients related factors gastric residence time [5]. Gastroretentive drug delivery has received significant interest in the past few decades as most conventional oral delivery system. The development of a long term oral controlled- release dosage forms has been difficult mainly because of the transit of the dosage forms through the gastrointestinal tract. The gastroretentive dosage forms have potential for use as controlled- release drug delivery systems and can overcome these problems by prolonging the retention time (Drugs remain in the gastric region for several hours) of a dosage form in the GI tract, thereby improving the oral bioavailability, reducing drug waste and improving solubility for drugs that are less soluble in a high pH environment. Diabetes Mellitus (DM) is a metabolic disorder characterized by hyperglycaemia, glycosuria, hyperlipemia, negative nitrogen balance, all these results into a wide spread pathological changes such as thickening of capillary basement membrane, increase in vessel wall matrix,and cellular proliferation resulting in vascular complication like lumen narrowing, atherosclerosis, sclerosis of glomerular capillaries, retinopathy,neuropathy and peripheral vascular insufficiency hence diabetes is one of the major causes of death and disability in the world.
Insulin is the most important regulatory hormone in the control of glucose homeostasis, consisting of 51 amino acids shared between two intramolecular chains (alpha and beta chain) secreted from beta cells of pancreatic islets. DM is of two type:
Type-I: Insulin dependent diabetes mellitus (IDDM)
DM occurs due to reduced or no secretion of insulin from beta cells of pancreatic islets which may be due to destruction of beta cells by autoimmune disease (type-IA/antibodies) or by some idiopathic disorder (type-IB/no antibodies) or drugs/chemicals/pathogens. Circulating insulin level are low or very low and patients are prone to Ketosis.
Type-II: Non insulin dependent diabetes mellitus (NIDDM)
There is no loss or moderate reduction in beta cells, insulin level is normal or high or low, the causes of DM may be
• Reduction in number of insulin-receptors (down regulation),reduced the sensitivity of peripheral tissues.
• Abnormally increased secretion of insulin and obesity
Nateglinide [N-(trans-4-isopropylcyclohexylcarbonyl)-D-phenylalanine] is a novel, highly physiologic, glucose regulator recently approved for the treatment of type-2 diabetes mellitus. Nateglinide has a rapid onset and short duration of insulinotropic action that results in reduction of glucose level. Nateglinide block the K-ion channels in pancreatic beta-cells, facilitating insulin secretion.
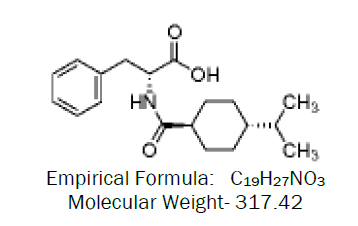
In the present investigation formulation and evaluation of nateglinide floating tablets, the basic idea behind the development of such a system is to maintain a constant level of drug in blood plasma. The tablet containing nateglinide drug float in the gastric fluid and slowly dissolves at a predetermined rate to release the drug and maintain the constant drug level in plasma
Nateglinide was received as a gift sample from Ind-Swift Laboratories Limited, Punjab (India). Ethyl cellulose (EC), polyvinyl pyrrolidone K-90 (PVP K90) were purchased from Himedia, Carbopol-934P, sodium bicarbonate, citric acid,magnesium stearate and talc were purchased from S.D Fine-chem ltd Ahmedabad, all other ingredients and solvents of analytical grade were purchase from local sources.
Experimental Design (32)
Factorial design is an experimental design technique, from which the factor involved and its relative concentration can be assessed. In the present study a 3² full factorial design was employed in which 2 factors were evaluated at 3 levels, experimental trials were performed at all possible 9 combinations. The independent variables selected for this study were concentration of ethyl cellulose (X1) and sodium bicarbonate (X2). % drug release for 30min,1h, 2h,4h,6h, 8h,12h, 16h,24h and floating lag time (FLT) were as dependent variables. Tablet weight was not constant for all batches because that does not require diluents which may causes variation in drug release profile.
Preparation of Nateglinide floating tablets
The formulation were fabricated using wet granulation method, the composition of different formulation of nateglinide floating tablet is shown in table 3. Ethyl cellulose (EC), Carbopol-934P, sod.bicarbonate and citric acid passed through sieve no.44 separately. All the ingredients (except mag.stearate and talc) were weighed accurately and mixed thoroughly for 20 minute using glass mortar and pestle. Granulation was done with sufficient isopropyl alcohol by passing wet coherent through a BSS # 18 sieve . The granules were dried in conventional hot air oven at 400C for 15 minute, dried granules were sieved through BSS # 22/44 mesh. then lubricated with mag stearate and purified talc and then compressed on a 11±0.1mm flat face single punch tablet machine.
Pre Compression Studies [5,6,7]
Bulk density (dB)
Density is determined by dividing weight of powder by volume of powder in g/cm3. Bulk denity is determined by weight of dry powder and the bulk volume in a graduated cylinder.
Tapped density (dT)
Tapped volume is measured by tapping of cylinder filled with bulk powder from a constant hight on flat horizontal surface for 100 times. This tapped volume gives tapped density by dividing weight of dry powder by tapped volume.
Hausner ratio
The Hausner ratio is a number that is correlated to the flowability of a powder or granular material. It is calculated by the formula HR= dT/dB . A Hausner ratio greater than 1.25 is considered to be an indication of poor flowability.
Carr index
It is also known as compressibility index. Carr index gives the important properties of powder or granules and is calculated by following equation
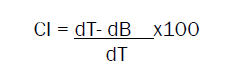
Angle of repose (̬̉)
It is calculated by fixed funnel method. The values obtained for angle of repose of all formulations were tabulated in table no.4. The values were found to be in the range from 48°.58Êù to 58°.01Êù this indicate poor flow properties of powder. The angle of repose is determined by using following equation
Ãâè =tanÃâ¹Ãâ°1 2H/d
Where H is height of funnel point , d is diameter of powder.
Post Compression Studies [5,6,7]
Weight variation test
Randomly selected 20 tablets were weighed individually and together in a single pan balance. The average weight was noted and standard deviation was calculated. Every individual tablet in a batch should be in uniform weight and weight variation in within permissible limits.
Shape of the tablet
Microscopic examination of tablets showed circular shape, flat face with no cracks.
Diameter & Thickness
Control of physical dimensions of tablets such as thickness and diameter is essential for consumer acceptance and tablet uniformity. The thickness and diameter of tablets were measured in mm using Vernier Calipers. Randomly selected 5 tablets were subjected for test and average thickness and diameter was calculated.
Hardness test
Hardness of tablets was determined by diametric compression by using Monsanto type hardness tester. Randomly selected 5 tablets were subjected for hardness test and average hardness was calculated.
Friability test
The friability of tablets was measured in a Roche friabilator (Camp-bell electronics, mumbai), randomly selected 20 tablets were taken, initial weight (Wo) of 20 tablets was noted then allowed for 100 revolutions in friabilator again taken the final weight (W) of 20 tablets. The percentage weight loss was calculated by
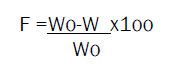
In-Vitro Buoyancy Study
In-vitro buoyancy of each formulation was determined by floating lag time(FLT) and total floatation time (TFT). Tablet of each formulation were individually placed in a 200 ml beaker containing 0.1N HCl solution at 37± 0.5°C. Time required for the tablet to rise to surface and float was FLT and the total time taken to remained buoyant without disintegration was TFT [8,9].(Table 6)
Drug Content Uniformity
The drug content in each formulation was determined by triturating 20 tablets and powder equivalent to average weight was added in 100 ml of pH 7.4 Phosphate buffer, followed by stirring 30 minutes. The solution was filtered through a 0.45μ membrane filter, diluted suitably and the absorbance of resultant solution was measured by spectrophotometer (UV-1700 pharmaspec, SHIMADZU). at 216 nm using pH 7.4 Phosphate buffer as blank [10,11]. (Table 7)
In-Vitro Drug Release Studies
The release rate of nateglinide from the floating tablets formulation F1 to F9 were determined by using the USP type II dissolution test apparatus (paddle type). The dissolution test was performed using 900 ml of pH 7.4 Phosphate buffer at 37± 0.5°C and 75 rpm. Ten-milliliter aliquots were withdrawn at time interval of 30 min, 1h,2h,4h,6h, 8h, 12h, 16h, 20h and 24h. The samples were replaced by their equivalent volume of pH 7.4 Phosphate buffer to maintain sink condition. The samples were analyzed at 216 nm by UV spectrophotometer (UV-1700 pharmaspec, SHIMADZU). . The percentage drug release was plotted against time to determine the release profile. The plot of cumulative percentage drug release versus time (hr) was plotted and depicted as shown in figure 1,2 and 3.
Process Optimization
Formulation of optimized batch F8 has been taken for the study of process parameters, the result showed that all optimized parameter were precise and they showed good results cumulatively in comparison to other formulation (F1 to F7 and F9) [12-14].
The powder thoroughly mixed with all ingredients are subjected to pre compression studies that the Hausner ratio (HR) varied between1.521 and 1.750, Carr index varied between 34.27% and 42.86% and angle of repose is varied between 48°.58Êùand 58°.01Êùwhich shows the poor flow property of powder, therefore wet granulation method was adopted for granulation. The assayed content of drug in various formulations varied between 97.78% and101.48% .Tablet buoyancy lag time varied between 06 sec and 20 sec, where as total floatation of each formulation is in between03h and 24h. The physical parameters hardness varied between 4.7 Kg/cm2and 5.8 Kg/cm2 and friability varied between 0.32% and 0.65%. The optimized formula F8 shows the constant release of drug for 24h and cumulative release of drug is 98.33%.
Nateglinide is a potent drug for treatment of diabetes mellitus type II but it has has a rapid onset and short duration of insulinotropic action. Gastroretentive formulation improves bioavailability, prolonged duration of action, reduced drug waste and dosing frequency. 32 full factorial design was employed in which 2 factors were evaluated at 3 levels, experimental trials were performed at all possible 9 combinations. Formulation of optimized batch F8 has been taken for the study of process parameters which shows the constant release of drug for 24h and cumulative release of drug is 98.33%.