e-ISSN: 2321-6190 p-ISSN: 2347-2294
e-ISSN: 2321-6190 p-ISSN: 2347-2294
Vanitha Priya D1*, Pandima Devi MK1, Arumugam P2, Sudharsan K3 and Anruradha V1
1PG and Research Department of Zoology, JBAS College for Women, Teynampet, Chennai, Tamil Nadu, India
2Armats Biotek Training and Research Institute (ABTRI), Chennai, Tamil Nadu, India
3Centre for Biotechnology, Anna Univeristy, Guindy, Chennai, Tamil Nadu, India
Received Date: 13/07/2016; Accepted Date: 15/02/2017; Published Date: 17/02/2017
Visit for more related articles at Research & Reviews: Journal of Zoological Sciences
This paper mainly focuses on using biopolymer as a potential larvicide. This work initially starts with extracting chitosan from the shrimp waste by a chemical method and it was characterized further. Chitosan is a natural carbohydrate biopolymer derived by deacetylation (DA) of chitin that is nontoxic, biodegradable, and biocompatible. But usually chitosan will not completely dissolved in water at normal conditions. Therefore, to increase the solubility and reaction rate, quaternized derivative of chitosan, N-(2-hydroxyl) propyl-3-trimethyl ammonium chitosan chloride (HTCC), is obtained and characterized. It highly dissolves in water and has more positive charge (quaternary ammonium groups) compared with chitosan. Next the larvicidal activity of the obtained HTCC against two mosquito species, Aedes and Culex, is evaluated. Next part of the work continues with synthesizing silver nanoparticles from the plant Euphorbia Antiquorum and characterized by UV Spec, FTIR, and SEM; then the larvicidal activity is examined similar to HTCC and the results obtained are compared with each other; Though both, silver nanoparticles and HTCC, show similar larvicidal activity, this study suggests HTCC is a better larvicidal agent having in mind the drastic effects of silver nanoparticles on aquatic ecosystem; Nanoparticles in aquatic systems are responsible for agglomeration and aggregation, dissolution, redox reactions and transformation into new solid phases, whereas chitosan and chitosan derivatives are completely degradable, biocompatible, and nontoxic to plants, animals, or human.
World health organization has declared mosquitoes as a public health pest throughout the world as they are responsible for the transmission of various dreadful disease-causing pathogens [1,2]. Though over 3,500 species of mosquitoes exist [3], only few of them are found to be awful, which act as vectors for a number of infectious diseases, belong to the genera Culex, Aedesand Anopheles.
Therefore, control over mosquito population is vital. It is more easier to control mosquitoes when they are in larval stages than controlling the dispersed adults [4] All the three stages of mosquito, i.e., egg, larvae, and pupa, need water for their growth and survival. Since a brood of larval mosquito can be killed while they concentrate in a pool of water. Using chemical pesticides such as DDT, organochlorines, organophosphates, methoprene, etc, were one of the methods followed in killing mosquitoes. Despite high cost, food safety concerns, and environmental hazardous [5,6], mosquitoes also develop genetic resistance [7,8] to these chemicals. Therefore, alternative vector control strategies, especially effective, environmental safe, biodegradable, low cost, and indigenous methods are extremely imperative [9-12]. Thereof, investigations and searching of natural and environmental friendly insecticidal substances are ongoing worldwide [13-15].
Biopolymers as Larvicidal Agent
Though silver nanoparticles are Eco synthesized, one of the effective methods widely used, they are researched to be responsible for biomagnification and heavy metal toxicity. Therefore, to overcome the issues of silver nanoparticle, biopolymer materials are used. One among the most applicable biopolymer is chitosan, extracted from the crustacean wastes, that has wellknown antibacterial and antifungal activities. More than that, after treatment they break down into monomers and at particular time completely disintegrates. Therefore, this work attempts to examine the larvicidal activity of N-(2-hydroxyl) propyl-3-trimethyl ammonium chitosan chloride (HTCC), synthesized from the chitosan extracted from shrimp shell wastes which is highly positively charged and easily soluble in water against two mosquito species Culex and Aedes. The obtained results are compared with the activity of silver nanoparticles synthesized from the plant Euphorbia antiquorum.
Synthesis of Silver Nanoparticles
Ten grams of fresh plant was washed and chopped in to pieces, later it was boiled in 100 ml deionized water for 10 minutes. The extract was then cooled and filtered using Whatman No 1 filter paper. 10 ml of this plant extract was added 90 mL of 1Mm silver nitrate solution. The solution was allowed to react at room temperature, after 24 h color change was observed from pale yellow to brick red. The reduction mechanism of silver ions in to nanoparticles was confirmed through the UV-visible spectrophotometer analysis at 420 nm. It is centrifuged at 13000 rpm and the pellet was air dried and collected in an air-tight eppendorf tube. Thus-obtained silver nanoparticles were characterized using UV-Vis spectrometer and SEM.
Extraction and Characterization of Chitosan and HTCC from Shrimp Waste
Ten grams of shrimp shell powder is mixed with 4% NaOH for 21 h and washed several times until neutral pH and dried at 60°C for 3 hours. It was followed by demineralization using 2% HCl for 2 h at 70°C. It was washed several times and the left out thus obtained is chitin. Thus-obtained chitin is DE acetylated using 60% NaOH for 72 hours. After deacetylation samples are cooled at room temperature and washed several times using distilled water until neutral pH, the remaining is the chitosan that is oven-dried completely for further use. HTCC was synthesized according to a known method [16].
Selection and Culturing of Mosquito Species
Mosquito species Aedes aegypti and Culex were selected for this study. Entire analyses were carried out against laboratoryreared vector mosquitoes which are free of insecticidal and pathogenic exposure.
Larvicidal Bioassay of Silver Nanoparticles
Larvicidal activity of silver nanoparticles was performed using a standard protocol of WHO. Synthesized nanoparticles were diluted to 5.0, 4.0, 2.0, 1.0, and 0.5 mg/l using double distilled water. Ten larvae of each species is added to each test solution with beakers containing 200 ml of water, and control is a beaker with distilled water. The mortality was recorded after 24 and 48 h and the experiment was repeated for four times for average value. Percentage mortality of both experiments was recorded using Abott’s formula.
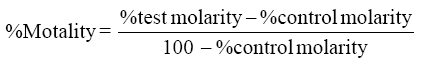
Larvicidal Bioassay of HTCC
Larvicidal bioassay of HTCC was carried out according to WHO standard procedures with slight modifications. Different concentrations ranging from 200 to 1000 ppm of HTCC were redissolved in 200 ml of tap water of 250 ml beakers and 10 larvae per concentration were used for all the experiments. A control was 200 ml of water. The number of dead larvae at the end of 24 and 48 h was recorded.
Characterization of Silver Nanoparticles
Color conformation: Change of the solution color from pale yellow to brick color after 48 hours initially confirms the formation of silver nanoparticles, as shown in Figure 1.
UV-VIS Spectrometer
Figure 2 shows the absorption spectra of AgNPs formed in the reaction media that have absorption maxima at 450 nm due to surface plasmon resonance of AgNPs confirmed the formation of silver nanoparticles.
SEM
Figure 3 shows the SEM of silver nanoparticles synthesized from Euphorbia Antiquorum. The silver nanoparticles were spherical in shape with particle size range from 5 to 40 nm and they are aggregated to each other.
Characterization of Chitosan and HTCC
Table 1 shows color, yield percentage, ash content, molecular weight, degree of deacetylation, solubility, water and oil absorbing percentage, pH and viscosity of the extracted and obtained chitosan and HTCC.
| S.NO | Properties | Chitosan | HTCC |
|---|---|---|---|
| 1 | Color | Creamy White | Creamy White |
| 2 | Yield | 21 | – |
| 3 | Ash content | 0.8% | – |
| 4 | Molecular weight | 10,197.49 | – |
| 5 | Degree of Deacetylation1` | 76% | – |
| 6 | Solubility | 35% | 98% |
| 7 | WBC% | 498.7% | 864.8% |
| 8 | OBC% | 356% | 665.9% |
| 9 | Ph | 8.9 | 7.2 |
| 10 | Viscosity | 298cps |
Table 1: Physical Properties of Chitosan and HTCC extracted from shrimp shell.
FTIR of Chitosan and HTCC
Figure 4 shows FTIR peak of chitosan. There is a broad band at 3438 cm-1 that is because of –NH2 groups, -OH groups, and intermolecular hydrogen bonds overlap each other. The peak at 2991 cm-1 represents the CH stretch [17]. Peak at 1639 cm-1 shows the presence of carbonyl group, which is due to incomplete deacetylation of chitin to chitosan. A significant peak is also observed at 1560 cm-1 indicates N-H bending of the primary amine.
Figure 5 shows FTIR of HTCC with a new peak at 1470 cm-1 represents the C-H bending of trimethylammonium group of HTCC. It should also be noted that the peak at 1560 cm-1 in chitosan is disappeared in HTCC due to the change of primary amine in chitosan to secondary amine in HTCC. The peak at 3430 cm-1 confirms the N-H stretching of a secondary amine and this peak proves the synthesis of HTCC by forming N-H group.
From the experiments carried out, Aedes was found to be resistive than Culex to both silver nanoparticles and HTCC and the rate of mortality was dose and time dependent.
Table 2 shows the larvicidal activity of silver nanoparticles on Aedes and Culex at 24 and 48 h, respectively. It can be seen that percentage of mortality increases with increasing concentration. In Culex, at least concentration, 0.5 ppm, the mortality was 10%; whereas at highest concentration, 5 ppm, mortality was 100%. In case of Aedes, it was 6% and 100% at 0.5 and 5pmm, respectively. Therefore, at all concentrations, Culex is more susceptible than Aedes.
| Concentration | Culex | Aedes |
|---|---|---|
| 24h | 24h | |
| 5.0 | 100% | 100% |
| 4.0 | 82 | 78% |
| 2.0 | 60 | 58% |
| 1.0 | 33 | 28 |
| 0.5 | 10 | 6 |
Table 2: Percentage Mortality of Silver Nanoparticles on Culex and Aedes at 24 h.
Table 3 shows the percentage mortality of HTCC against Aedes and Culex. As before, Culex is more susceptible than Aedes and mortality increased with increasing concentration. Culex showed 12% mortality at low concentration and 100% at highest concentration. Aedes showed 10% mortality at low concentration and 100% at highest concentration, respectively. Both Silver nanoparticles and HTCC showed 50% mortality at 2 ppm and 600 ppm, respectively.
| Aedes | Culex | |
|---|---|---|
| Concentration | 24h | 24h |
| 5.0 | 100% | 100% |
| 4.0 | 82 | 70% |
| 2.0 | 54 | 50% |
| 1.0 | 28 | 20 |
| 0.5 | 10 | 10 |
Table 3: Percentage Mortality of HTCC on Culex and Aedes at 24 h.
Table 4 shows lethal concentration (LC50 and LC90) values of the silver nanoparticles and HTCC on Culex quinquefasciatus and Aedes aegypti. The LC50 values of silver nanoparticles and HTCC on Culex quinquefasciatus were 1.179 and 2.212, respectively; 2.392 and 2.097, respectively, for Aedes aegypti. The LC90 values are 4.555 and 4.521 for Culex and 5.129 and 4.428 for Ades treated with Silver nanoparticles and HTCC, respectively, and the chi-square values are significant at p.
| Mosquito species | Treatment | LC50 (ppm) | 95% confidence limit | LC90 (ppm) | 95% confidence limit | Intercept±SE | Slop pt±SE |
χ2 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| LL | UL | LL | UL | |||||||
| Culexquinquefasciatus | Silver nanoparticles | 1.179 | 0.837 | 1.456 | 4.555 | 3.812 | 6.001 | 3.6±0.26 | 3.95±0.56 | 6.9* |
| HTCC | 2.212 | 1.882 | 2.532 | 4.521 | 3.810 | 5.880 | 3.57±0.27 | 4.12±0.58 | 5.1* | |
| Aedesaegypti | Silver nanoparticles | 2.392 | 2.034 | 2.749 | 5.129 | 4.243 | 6.954 | 3.53±0.27 | 3.86±0.56 | 7.3* |
| HTCC | 2.097 | 1.766 | 2.414 | 4.428 | 3.711 | 5.811 | 3.73±0.25 | 3.94±0.56 | 5.4* | |
Table 4: Lethal concentration (LC50 and LC90) values of the silver nanoparticles and HTCC on Culex quinquefasciatus and Aedes aegypti.
Larvicidal activity of silver nanoparticles was already reported by literature [12-16] Besides the larvicidal activity, negative impact of silver nanoparticles on the aquatic life after treatment is more concerned. The most important processes affecting the fate of nanoparticles in aquatic systems are agglomeration and aggregation, dissolution, redox reactions and transformation into new solid phases [18-22] though used in very less quantity. The use of nanomaterials and their potential environmental and human health risks [23] are of increasing concern and social debate [16,24] and have been the subject of many government reports.
There are several reports evidenced the drastic effects of nanoparticles on aquatic environment. Julia Fabrega, et al. reported that concentrations of Ag NPs, as low as, just a few ng/L, can affect prokaryotes, invertebrates, and fish and also they also studied the mechanisms of toxicity. researched Ag ion toxicity in vivo in freshwater fish species with LC10 values as low as 0.8 μg/ L had effect on certain freshwater fish species [25].
Therefore, completely nontoxic biocomponent is indeed for treating mosquito larvae which reside in water bodies. Chitosan is a polysaccharide which forms the basis of the main constituent of the outer skeleton of insects and crustaceans like shrimp, crabs, and lobster [26]. There are several studies evidenced the antimicrobial activity of chitosan [27,28] and also reported that chitosan has effective antifungal activity which inhibits spore germination, germ tube elongation, and radial growth. Few works also evidenced antiprotozoal activity of chitosan [15,29]. The killing mechanism is due to the interaction mediated by the electrostatic forces between the protonated NH+3 groups and the negative residues [30], presumably by competing with Ca2+ for electronegative sites on the membrane surface of the microbes.
To improve further the solubility of chitosan in water, various derivates of chitosan were prepared. Among the various chitosan derivatives, the derivatives with quaternary ammonium groups have shown higher efficient activity against microbes compared to those of chitosan [31,32]. This may be due to enhanced positive charged quaternary amine group, known to targeted on the negatively charged cytoplasmic membrane of microbes, altering membrane properties and impeding nutrients entering the cells [33,34]. Therefore, same mechanism may occur in killing mechanism of mosquito larvae [35-40].
From the results it can be concluded that HTCC has equivalent and better larvicidal activity when compared with silver nanoparticles [41-46], which shows 100% mortality 1000 ppm in both Aedes and Culex. And the activity can also further enha nced by synthetizing HTCC nanoparticles [47-50].
This study compares the larvicidal activity of three silver nanoparticles and HTCC. Despite equal efficacy, this study suggests using HTCC as a larvicidal agent is advisable and more beneficial; because chitosan is completely degradable, biocompatible, and nontoxic to plants, animals, or human. This study can be further extended in future by synthesizing HTCC nanoparticles and examining its larvicidal activity. This work also aims to explore the parasitic activity of HTCC in future studies.
Author would like to thank Armats biotec for providing the lab facilities to carry out the research and also would like to thank Department of Environmental Technology, Central Leather Research Institute, Guindy for proving the instrumentation facilities for this research.