ISSN ONLINE(2320-9801) PRINT (2320-9798)
ISSN ONLINE(2320-9801) PRINT (2320-9798)
Suganya.V1
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Computer and Communication Engineering
Electrocardiogram (ECG) is the P, QRS, T wave representing the electrical movement of the heart. The fine changes in amplitude and interval of ECG cannot be interpreted exactly by the naked eye, hence imposing the need for a computer assisted diagnosis tool. Automatically classified five types of ECG beats of MIT-BIH arrhythmia records. The five types of beats are Normal (N), Right Bundle Branch Block (RBBB), Left Bundle Branch Block (LBBB), Atrial Premature Contraction (APC) and Ventricular Premature Contraction (VPC). We have match up to the performances of three approaches. The first approach apply principle components of segmented ECG beats, the second approach apply principle components of error signals of linear prediction model, whereas the third approach apply principle components of Discrete Wavelet Transform (DWT) coefficients as features. These features from three approaches were independently classified by feed forward neural network (NN) and Least Square- Support Vector Machine (LS-SVM). To attain the highest accuracy with the first approach using principle components of segmented ECG beats with average sensitivity of 99.90%, specificity of 99.10%, PPV of 99.61% and classification accuracy of 98.11%. The system built-up is clinically set to arrange for collection screening programs.
Keywords |
| Electrocardiogram, MIT-BIH arrhythmia database, Discrete Wavelet Transform (DWT), Neural network (NN), Support Vector Machine (SVM). |
INTRODUCTION |
| Cardiac arrhythmia is a grouping term for a heterogeneous collection of conditions in which there is irregular cardiac electrical activity. Arrhythmias are usually encountered in clinical practice, arising from chaotic electrical activity. Many arrhythmias are similar to ventricular fibrillation and flutter is life threatening medical emergencies that can result in cardiac capture hemodynamic collapse and sudden death. Cardiac arrhythmias happen most often in individuals with an basic cardiovascular disease like hypertension, coronary artery disease and cardiomyopathy. They happen due to a deficiency in impulse formation or impulse conduction or due to both. |
| The electrocardiogram (ECG) is being normally used as a diagnostic device to decide different types of arrhythmias. Perfect and early recognition and differentiation of arrhythmias is important, especially for fatal and life threatening arrhythmias like ventricular tachy-arrhythmias that can effect in sudden cardiac death. Many types of arrhythmias, such as atrial fibrillation, are related with an improved possibility of thromboembolic events and stroke. The inbuilt complication and mechanistic and clinical interrelationships of arrhythmias repeatedly brings about diagnostic problems for treating physicians and primary health care professionals creating frequent misdiagnoses and cross classifications using visual criterion. Computer- aided cardiac arrhythmia detection and categorization can take part in a important role in the management of cardiovascular diseases. The modern computerized algorithms can recognize cardiac arrhythmias with privileged diagnostic correctness with considerable drop in the expenditure. |
| A minicomputer scheme was planned to analyze three types of beats (Normal, Supraventricular ectopic beats (SVEB) and ventricular ectopic beats (VEB)) by means of time domain characteristics in a 24 hour ambulatory ECG. Linear forecast process was used to identify Ventricular Premature Contraction (VPC) with a sensitivity of 92%. Time domain characteristics of Normal, SVEB and VEB were modelled using Hidden Markov Model. The ECG morphology beside with RR interval characteristics be used for the categorization of Normal and five types of arrhythmia beats using particle swarm optimization method and achieve an average accuracy of 93.27%. Normal, Atrial Premature Contraction (APC), Supra Ventricular Tachycardia (SVT), Ventricular Tachycardia (VT), VPC and Ventricular Flutter (VF) ECG beats were classify with autoregressive model and generalized linear model classifier with an accuracy of 93.2%. Principal components of two kinds of abnormal ECG beats were classify with Gaussian Mixture Model (GMM) with an exactness of other than 94%. Normal, congestive heart failure, Ventricular Tachycardia and atrial fibrillation beats of ECG were classify with multilayer perceptron neural network and characteristic saliency and obtained 97.78% of exactness. |
| The independent components arrangement strategy was planned with Independent Component Analysis (ICA) and classify eight types of ECG beats (Normal, Left Bundle Branch Block, Right Bundle Branch Block, Ventricular Premature Contraction, Atrial Premature Contraction, paced beats, Ventricular Flutter wave and ventricular ectopic beats) via support vector machine and probabilistic neural network and reported more than 98% of accuracy. Six types of ECG beats (Normal, Ventricular Premature Contraction, fusion of ventricular and Normal beats, Atrial Premature Contraction, Right Bundle Branch Block, fusion of paced and Normal beats) were classify via particle swarm optimization and radial basis function neural network classifier and achieved more than 95% of sensitivity and more than 98% specificity. Although many mechanism were reported on arrhythmia beat classification, there is a need to progress the categorization precision when used for enormous database. Also most of the methods reported use composite mathematical description having presence lot of computational trouble while evaluate these description. To extend a simple attitude for arrhythmia beat classification, with maximum diagnostic exactness level as used for bulky record. |
II. METHODOLOGY |
| Fig.1 depict the planned methodology of categorization of Normal and four types abnormal beats in the ECG of arrhythmia. |
2.1 Preprocessing and segmentation |
| The ECG signal downloaded from MIT-BIH arrhythmia database may contain artifacts, noise and baseline wander. Thus it is essential to denoise the ECG signal to take out all these useless parts of the signal. First the raw ECG signal was subjected to wavelet based denoising using daubuechies-6 wavelet. The ECG signal was decomposed upto six levels of decomposition. The sixth level estimate sub band consists of frequency band of 0–2.8125 Hz, which primarily consists of baseline wander. Therefore this sub band is not essential. The ECG will not include much information after 45 Hz. The essential sub bands are detail coefficients of 3rd, 4th, 5th and 6th levels. So only these coefficients are taken and all other sub band coefficients are replace with zeros and inverse wavelet transform is computed to obtain denoised ECG. After denoising the ECG, the QRS complex can be recognised. The QRS complex is an significant tip in the ECG signal, also it is simple to identify by signal processing algorithms due to its sharp and well-known shape. It consists of computation of derivatives, moving window integrator, squaring and detection of rising edge of pulses. The derivative provide the gradient information of ECG waveform, squaring will provide higher amplitudes and suppress smaller amplitudes and moving window integrator performs averaging operation, so removes noise. After identification of QRS complex, 99 samples were selected from the left side of QRS mid-point and 100 samples after QRS mid-point and the QRS mid-point as a part or beat of 200 samples. |
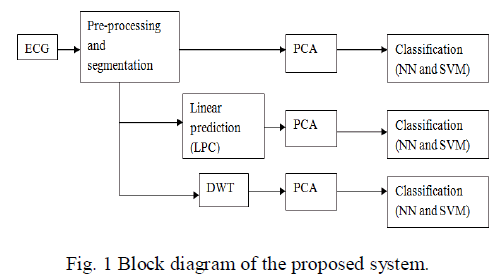 |
2.2 Linear prediction |
| In linear prediction (LP) analysis, the ECG signal part, x(n) is modeled as a linear arrangement of its past input signals x(n-k), k = 1,2,. . . ,p where p refer to the order of calculation and a(k) is the kth linear calculation coefficient. The error in calculation is given by |
| e(n)=x(n)-y(n) (1) |
| where y(n) is the predicted signal, given by |
| y(n)= (2) |
| Substituting Eq. (2) into Eq. (1) and taking Z transform, one attain |
| -k=A(z) (3) |
| When the signal x(n) is fed to the system defined by the transfer function A(z), the Residual Error Signal (RES), e(n) is obtained and if signal e(n) is accepted through the system 1/A(z), we get the reconstruct original signal y(n). The ECG signal segment x(n) is predicted from the third order linear predictor using the coefficients, a(k). The RES represent the element of the ECG which could not be predicted using LP model. The output of linear predictor is arbitrary in nature. In order to compute LP coefficients, Levinson–Durbin recursion is used. Another time the output of LP filter has same number of samples as that of the input (200 dimensions). But RES signal is not related to itself. The 200 samples of RES are used for consequent analysis. |
2.3 Discrete wavelet transform |
| The wavelet transform has the ability of providing resolution concurrently in time and frequency. Due to energy compaction property of wavelet transform, it can provide sparser representation than time domain. |
| To decompose a ECG signal beat into time-frequency components, a basis function at level a and setting b is defined as, |
| a,b(t)= (4) |
| The wavelet transform is sampled on a dyadic grid scale to get discrete wavelet transform, such DWT at scale 2-m and time instant n is given by, |
| m,n(t)=2-m/2 -mt-n) (5) |
| Using the dyadic wavelets, the DWT can be written in conditions of the inner result between the signal and the wavelet basis function as, |
| m,n= m,n(t)dt (6) |
| The inverse discrete wavelet transform is computed as, |
| m,n m,n(t) (7) |
| In order to decompose ECG into DWT, the shape of the wavelet basis function should be related in morphology to the ECG. |
2.4. Principle Component Analysis (PCA) |
| The Principle Component Analysis (PCA) is a linear dimensionality reduction technique, that provides projection of the data in the directions of highest variance. The PCA algorithm involves working out of covariance matrix from the collection of ECG beats, eigenvalue and eigenvector disintegration of covariance matrix, sorting eigenvectors in the downward order of eigenvalues and finally projecting the original ECG data in the directions of sorted eigenvectors. The first few components will be the most of the inconsistency present in the data. The first 12 components are used behind PCA for pattern classification. |
III. CLASSIFICATION |
| A fully connected feed forward neural network (NN) and support vector machine are used for pattern identification. |
3.1. Neural network |
| A fully connected feed forward neural network is used for automatic pattern identification. The neural network consists of a layer of input neurons, two layers of unknown neurons and one layer of output neurons. Random weights are primarily thought and the training data is fed to the neural network to attain network response. Based on the exercise labels the discrepancy between the obtained output and desired output by the network is computed and it is called as error signal. Based on this error signal, the weights are efficient by back propagating the error. The process is frequent until the training error is below a given threshold. Later the testing data is fed to the qualified neural network and the classified output is noted and the act of classification is computed based on testing labels. |
3.2. Support Vector Machine |
| Support Vector Machine (SVM) is a well non linear and single layered network which is having higher simplification capability that it can classify hidden patterns correctly. The classifier minimizes structural hazard instead of experimental risk as in other classifiers. It maximizes the expanse between the patterns and the class separating hyperplane simultaneously in order to separate the patterns belonging to different classes. Usually the patterns in the specified aspect space are not linearly independent, therefore they are anticipated into a high dimensional space where the features are held to be linearly separable and classification is performed. The system is called kernel trick. Usually used kernels are linear, quadratic, polynomial of order 3 and radial basis function (RBF) kernels. |
RESULTS |
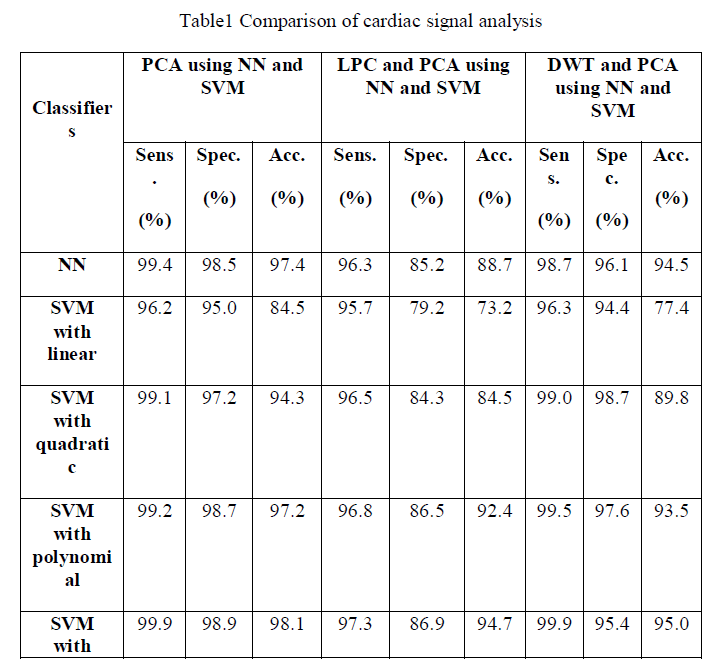
| The ECG signals from MIT-BIH database were first denoised using wavelet based denoising technique. After the denoising, it was subjected to QRS complex detection. After detection of QRS complex, the ECG was segmented to obtain 200 samples segment as a beat for consequent analysis. Three approaches were used. In the first approach the segmented ECG signal was used for its dimensionality drop using PCA. The dimensionality reduced principle components were used for the pattern classification of five types of beats in arrhythmia. In total 12 components were used for the pattern classification using feed forward neural network and SVM. |
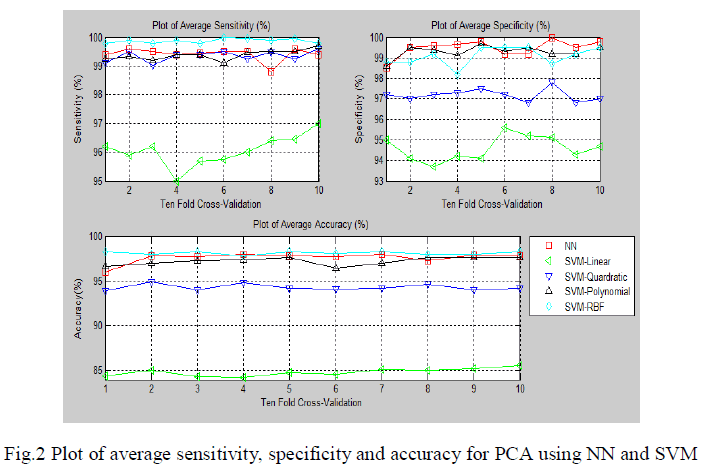 |
| The fig.2 show the average sensitivity, specificity and classification accuracy using different classifiers for various folds of ten-fold cross validation respectively.In the first approach the segmented ECG signal was used for its dimensionality reduction using PCA. The dimensionality reduced principle components were used for the pattern classification of five types of beats in arrhythmia. |
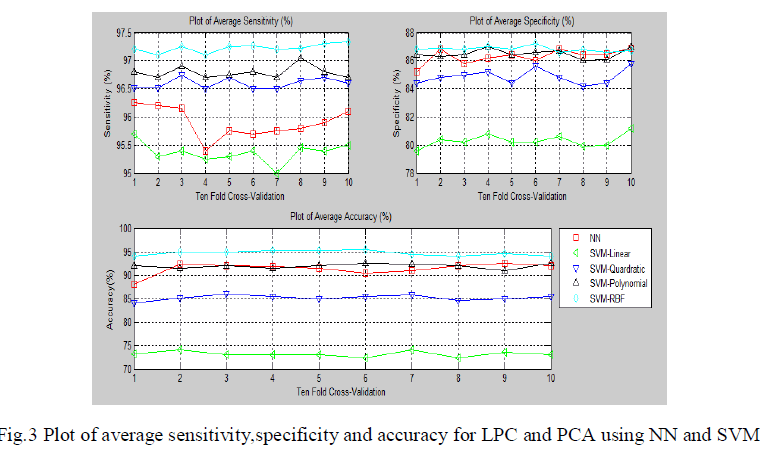 |
| The fig.3 show the average sensitivity, specificity and classification accuracy using different classifiers and for each of the ten-fold cross validation respectively.The second methodology involves computation of linear predictive coefficients from ECG beats. Based on the linear predictive coefficients, the error signal of the linear prediction model is derived. Since there will be 200 samples per beat of ECG in the error signal, to reduce its dimensionality, PCA was used. |
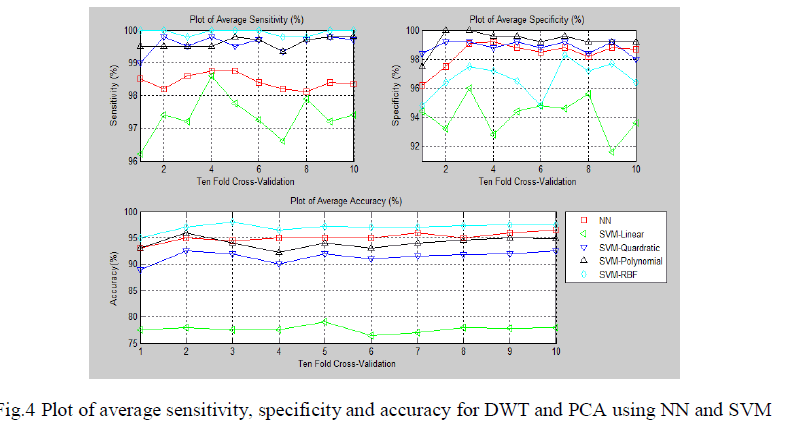 |
| The fig.4 show the sensitivity, specificity and accuracy using different classifiers for various folds of ten-fold cross validation respectively. It involves the computation of DWT coefficients. |
 |
 |
V. DISCUSSION |
| The ECG beat classification using PCA features in time and DWT domains. Four types of ECG beats (Normal, supra-ventricularectopic, ventricular ectopic and fusion beats) were classified using mixture of experts approach and reported 94% of accuracy. The Hermite function decomposition coefficients were used for 25 types of arrhythmia beats classification using Self Organizing Maps (SOM) and achieved 98.51% of accuracy. The Higher Order Spectra Analysis (HOSA) features were used to classify seven types of cardiac beats using ECG using fuzzy hybrid neural network classifier and achieved 96.06% of accuracy. SVEB and VEB beats were classified using a patient adapting heart beat classifier and obtained accuracy of 95.9% and 99.2% respectively. The Hermite transform coefficients and time intervals between two neighboring R-peaks of ECG signals were used as features for block based neural network and classified five types of ECG beats. |
| To have compared the performance of three approaches: (i) principle components of segmented time domain ECG beats, (ii) the principle components of error signals of linear prediction model of ECG beats and (iii) the principle components of discrete wavelet transform sub bands. The first method performance yielded an highest average classification accuracy of 98.11%, average sensitivity and specificity of 99.90% and 99.10% respectively compared to the other two methods. |
VI. CONCLUSION |
| ECG signal can be used as a reliable indicator of heart diseases. In this work, PCA analysis is effectively used as a non-invasive tool for the classification of five types of cardiac classes. In this study, we have compared the performances of three methodologies. The first technique uses principle components of segmented ECG beats, the second technique uses principle components of error signals of linear prediction model, and the last technique uses principle components of DWT coefficients as features. The first method uses principle components of segmented ECG yields an highest average accuracy of 98.1%, sensitivity and specificity of 99.90% and 98.9% respectively. The proposed methodology has provided improved results and can be used in practical arrhythmia monitoring systems. Our proposed methodology has immense applications in electronic cardiac pacemakers, remote patient monitoring and in intensive care units. |