Institute des Sciences Moléculaires, UMR 5255, Univ. Bordeaux, 351 crs de la Libération, 33400 Talence, France
Received date: 21/04/2015 Accepted date: 23/06/2015 Published date: 28/06/2015
Visit for more related articles at Research & Reviews: Journal of Medicinal & Organic Chemistry
A lot of studies have evidenced the beneficial effects of flavonoids in diseases linked to oxidative stress. Radical scavenging is one of the possible ways of action through two processes: transfer of hydrogen radical or electron. The enthalpies corresponding to these two processes can be calculated with a good accuracy to evaluate the radical scavenging properties of polyphenols. If a lot of studies have determined the radical scavenging properties of aglycone or glycosylated flavonoids, no theoretical calculations have been performed on the metabolites of these compounds that are the active molecules in tissues or in human plasma. In this paper, the enthalpies corresponding to the two radical scavenging processes, transfer of a hydrogen atom or an electron, are accurately calculated for the metabolites of two abundant flavonoids, quercetin and kaempferol and the evolution of radical scavenging properties consecutive to the metabolism is discussed, giving some insight in the real active radical scavengers in the body.
Flavonols, bond dissociationenthalpies, electron transfer enthalpies, metabolites, antioxidants
A lot of scientific studies support a protective effect of polyphenols on chronic degenerative diseases [1]. Initially this effect was attributed to the radical scavenging properties of these compounds but nowadays a more complex pattern emerges. Concomitantly with the radical scavenging properties, the in vitro effects exhibit a diminution of oxidative stress by other pathways, in particular metal interaction and inhibition of ROS producing enzymes such as xanthine oxidase, NADPH oxidase and lipoxygenase [2]. There is also evidence of processes where polyphenols act as signaling molecules. In this pattern, the interaction decreases the inflammatory response of the bodies and thus, the production of ROS. However the in vivo studies are not simple to analyze and can be in contradiction with the in vitro ones. This is due to the fact that polyphenols are metabolized during their absorption in the body. The active molecules are no more the ingested ones.
It has been established that the dietary intake of polyphenols ranges between 0.15 and 1 g a day [3]. Among the polyphenols, the flavonoids have paid a lot of attention because of its numerous properties. The consumption of some of them, generally as glycosylated derivatives, can attain 10-100 mg a day. However their levels rarely exceed 1 mM in human plasma. This is the consequence of a strong and complex catabolism during digestion that has been extensively studied by both in vitro and in vivo experiments.
Catabolism can occur in all the digestive apparel and most of the glucosides are deglycosylated by specific enzymes before the absorption. Then, other enzymes metabolize them. The biotransformation of polyphenols is very complex and is specific of the part of the digestive apparel where it occurs as illustrated by a recent study [4]: it has followed in vitro the metabolism of apple polyphenols and in particular, of glycosylated quercetins, in the different juices of the digestive apparel. It has evidenced that hydrolysis of substituted quercetins in the mouth is not due to the saliva but depends on oral bacterial flora. Flavonols are stable in simulated gastric juice. In duodenal juice, the flavonol glycosides were found stable but the authors observed that aglycone quercetin is almost completely degraded within 2 hours in 3,4-dihydroxybenzoic acid and phloroglucinol through the intermediate 2,4,6-trihydroxybenzoic acid. In contrast, quercetin has been found stable in ileostomy incubations but the flavonoids glycosides can be hydrolyzed. After absorption, the metabolites can be modified in the liver. In order to study such process, the authors have incubated the molecules in freshly isolated hepatocytes: aglycone quercetin underwent intensive conjugation reactions, principally in 4’-O-glucuronide. To sum up, the authors’ findings correlate with other studies: an important metabolism of aglycone occurs in duodenum and intestine. The glycosides are better absorbed by intestine but are further metabolized in the liver. The most common conjugates found in the plasma are glucuronates, sulfates or methylated compounds [5,6].
Other studies have emphasized the importance of the biotransformation of polyphenols in the colon. The biotransformation of quercetin and its glycosylated derivatives is very important since only a small part (1-5%) of the originally consumed quercetin is excreted from the digestive bolus of the colon [7]. An important part of this metabolism is due to the microflora contained in the gastro-intestinal tract, and in particular in the distal gastro-intestinal tract. Each portion of the gastro-intestinal tract has a distinctive flora, but the concentration of bacterial species is much more important in its distal part. The transformations due to the microflora are O- and C-deglycosylation, hydrolysis of esters and deglucuronidation, aromatic dehydroxylation, demethylation and oxidation of the aliphatic elements generated following the rupture of aromatic ring. There are different possibilities for the biotransformation of flavonoids: fission of the A-ring (Figure 1) has been observed with the ultimate metabolite CO2. Eubacterium spp. degrades flavonoids with a deglycosylating activity and the ability to split the C-ring. Some studies [8,9] demonstrated that other bacteria Clostridium spp. rapidly cleave the C-ring of glycosylated or aglycone quercetin with formation of 3,4-dihydroxyphenylacetic acid and presumably phloroglucinol. The first molecule can also be further metabolized in 3-hydroxylphenylacetic acid and 4-hydroxy- 3-methoxyphenylacetic acid. 3,4-dihydroxytoluen is also considered as a metabolite of quercetin. For most of the flavonoids, the phenolic acids originated from the B-ring retain it intact. Other metabolites derive from the A-ring. The importance of the metabolism in the colon can be evaluated by in vivo studies with volunteers who had their colon removed surgically. The ileal fluid of these subjects contains 86% of the quercetin-3-O-rutinoside that they have ingested [10] and no metabolites are detected in their plasma or urine. In particular, no phenolic acid originated from the C-ring cleavage can be evidenced in the urine. By comparison, in healthy volunteers, the amount of these phenolic acids corresponds to 22% of the flavonoid intake.
Due to the importance and complexity of metabolism of flavonols and other polyphenols, it is important to know if the metabolites retain the radical scavenging properties of the ingested compounds. The aim of this paper is to calculate the radical scavenging properties of the metabolites of two abundant flavonols in order to assess the possibility of radical scavenging in vivo.
Quercetin is a very common flavonoid which can be found in a lot of vegetable and fruits. Foods rich in quercetin include black and green tea (Camellia sinensis; 2000–2500 mg/kg), capers (1800 mg/kg), lovage (1700 mg/kg), apples (440 mg/kg), onion, especially red onion (191 mg/kg), red grapes, citrus fruit, tomato, broccoli and other leafy green vegetables, and a number of berries [11] . Kaempferol is another aglycone flavonoid which possesses less antioxidant properties. It can be isolated from tea, [12] broccoli, Delphinium, Witch-hazel, grapefruit, cabbage, kale, beans, endive, leek, tomato, strawberries, grapes, brussels sprouts and apples. These two flavonols are differently metabolized in the intestine and liver. Kroon et al. [13] have reviewed the structures of different polyphenols in human plasma and urine. Quercetin is found as a mixture of glucuronides and sulfates of quercetin and methyl quercetin. The catabolism of kaempferol is less important since there is evidence of only free kaempferol and kaempferol- 3-glucuronide in human plasma. In this paper, the radical scavenging properties of these different metabolites are calculated for a comparison with the aglycone compounds. The molecules resulting from the cleavage of the C-ring or B-ring have also been investigated since a large amount of the aglycone flavonols are metabolized by the microflora of the gastro-intestinal tract.
Computational methods
It has been shown [14] that in protic solvents and water, essentially two mechanisms lead to the scavenging of a free radical by a flavonoid. In the first one, the free radical gains a hydrogen atom from the antioxidant, which becomes a radical:
R• +ΦOH → RH +ΦO• (1)
The occurrence of such a hydrogen transfer (HAT) is determined by the bond dissociation enthalpy (BDE) that measures the strength of the OH bond.
Relation (2) gives the enthalpy corresponding to such process:
BDE = H(ΦO•) + H(H• ) − H(ΦOH) (2 )
where H(ΦO• ) is the enthalpy of the phenoxyl radical, H(H• ) is the enthalpy of the hydrogen radical and H(ΦOH) that of the flavonoid. If the BDE of the polyphenol is lower than that of RH, the reaction is exothermic.
If the polyphenol molecule exists in anionic form at physiological pH because of its low pKa, the second mechanism has been named Sequential Proton-Loss Electron-Transfert (SPLET): [15,16].
ΦOH ↔ ΦO-+ H+
ΦO-+ R•→ ΦO•+ R-
The significant enthalpy related to this reaction can be denoted ETE (electron transfer enthalpy) [17]. In this paper, the ETE is calculated by:
ETE = H(ΦO• ) − H(ΦO− ) (4)
The ability of the polyphenol to scavenge the radical depends on the relative values of the ETE of R− and ΦO− Thus, electron or radical hydrogen donations are two independent processes that depend on ETE or BDE values of the radical to scavenge. However, if the BDE or ETE values of the polyphenol are low, their ability to scavenge relatively stable radicals will be increased.
The enthalpies corresponding to the two above mentioned mechanisms are calculated by using the Gaussian 09 package [18]. The calculations are performed with density functional theory (DFT) method and the hybrid functional B3LYP which is largely used for such calculations because of its good reproduction of experimental results when it is combined with a large basis set. The procedures for the calculations of the two thermodynamic quantities characteristic of the donation processes are the following:
BDE:
The enthalpy of a species FOH is estimated as:
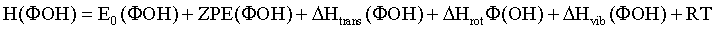 (5)
(5)
where E0 is the calculated total electronic energy, ZPE the zero point energy, and the other terms the thermal contributions of the enthalpy originating from the translation, rotation or vibrations of the molecule. The five last terms are evaluated in frequency calculations performed by Gaussian.
The geometries of the whole molecules and radicals are optimized within the density functional theory (DFT) approach with the B3LYP exchange correlation potential and the basis set: 6-31+G(2d,2p). The unrestricted open-shell approach (UB3LYP) is used for radical species. The enthalpies H(FOH) and H(FOÃâ¹Ãâ¢) are evaluated at the same level of calculations. The BDE corresponds to enthalpy difference between the two species minus the enthalpy of the hydrogen radical. As in reference [19] the enthalpy of the HÃâ÷ radical has been taken equal to 0.49764 hartrees. The enthalpies are calculated within the frame of the self-consistent reaction field polarized continuum model (SCRF-PCM) [20,21] that mimic the solution in solvents by building a cavity of overlapping spheres. Thus, the BDEs have been calculated in water to give more accurate quantities since the BDEs can be slightly different for a few molecules in the gas phase or in solution in water.
ETE: For the calculation of the electron transfer enthalpies, the geometries of the anion and radical are optimized with the same method and basis set B3LYP/6-31+G(2d,2p) (UB3LYP/6-31+G(2d,2p)). The zero point energies (ZPE) are calculated within the same level of calculations. The ETE are the difference between energies corrected from the ZPE. Contrary to the BDE, the ETE are completely different in solution or gas phase. The solvent effects are evaluated in the framework of self-consistent reaction field polarized continuum model (SCRF-PCM) [20,21] implemented in Gaussian package.
The results of the calculations are summarized in Table 1. This table displays the BDE of the metabolites in both neutral and anionic forms, the ETE and the free energy difference between the anionic and neutral forms DE. The former quantities, BDE and ETE are given in kcal/mol, the later in hartrees. The energy difference DE gives an evaluation of the pKa of relative compounds [22] by comparison with known products. Quercetin has two types of conformations [23] according to the direction of the OH bonds of the catechol moiety. When the catechol points toward the position 3, with the OH bond at the position 3’ toward the OH bond at 4’, the deprotonation site is the position 4’. When the conformation of the catechol is such that the 4’ OH bond points toward the position 3’, the deprotonation site is the position 7. This conformation is slightly more stable but has a higher DE. It can be used as a reference with a pKa near 7.
Table 1: Calculated BDE, ETE (in kcal/mol) and DE (in hartrees) for quercetin, kaempferol and their metabolites in water. The DE corresponds to the energy difference between the molecule and the anion. The numbers in parenthesis correspond to less stable conformations which have a non-negligible concentration at room temperature.
Substituted flavonoids: The substitution by a methyl, a sulfate or a glucuronic acid of one hydroxyl group hydrogen atom in quercetin increases the BDE by four to eight kcal/mol. This result was expected when the catechol is substituted since quercetin predominantly gives the hydrogen at position 4’. However, in deprotonated aglycone compound, the BDE of position 3 has the same order of magnitude than that of position 4’. If the BDE were a local property of hydroxyls, the substitution would not have such effect. The substitution at position 3 or 3’ lower the BDE of the remaining hydroxyls, illustrating once more that the value of BDE is not only linked to the position of the OH group in the molecule but also to the possibility of electronic delocalization [24]. The most powerful substituted radical scavenger is quercetin-3’-o-methyl in its anionic form. However, its evaluated pKa seems relatively high and this form is unlikely to exist at physiological pH. Acids substitute the other metabolites. Thus, they are anions at physiological pH. The DEs of the dianions have been calculated to evaluate if a second deprotonation is possible. Only one conformation of quercetin-3-o-glucuronide possesses a DE of the same magnitude as quercetin. Quercetin-3-o-glucuronide has four conformations of approximately the same free energy. These conformations differ by the orientation of the hydroxyl groups in the two positions 7 and 3’4’. When the hydrogen atoms of the catechol group point toward the 2’ position, the calculations indicate that the deprotonation occurs at position 7 (Figure 2). The calculated DE is 0.465 hartrees, which is high, compared to that of quercetin. When the cathechol group points in the other direction, the deprotonation occurs at the 4’ position and the calculated DE is of the same magnitude as for aglycone quercetin. This dianion has a low ETE and can be considered as one of the best radical scavenger of the series of substituted quercetin. Quercetin-3’-o-sulfate has only two stable conformations: in these conformations, the hydroxyl group at the position 4’ points toward the sulfate. The deprotonation occurs at the position 7 and the calculated DE is relatively high. On the other hand, quercetin-3-o-sulfate possesses the four configurations of quercetin-3-oglucuronide. But the DE of the anion deprotonated at the position 4’ is two high for the conformer to be dissociated at physiological pH.
In summary, the metabolites of quercetin with one substituent possess BDE between 75 and 79 kcal/mol and can be considered as moderate antioxidants by donation of hydrogen radical. One can notice that these values of BDE are sufficiently low to permit the scavenging of the peroxyl radical in lipid media. However the ability to give an electron of these molecules is negligible with one exception: the dianion of quercetin-3-o-glucuronide. The disubstituted metabolite quercetin-3-o-glucuronide-3’- methyl has lost all the radical scavenging properties of the parent compound. One can also observe such a loss for the metabolite of kaempferol.
It is known that glycosylation has the effect to decrease the BDE of flavonoids when the substitution is situated on the catechol or at the position 3. It has been observed that this decrease of the BDE was the consequence of the decrease of the mulliken charge on the oxygen atom of the hydroxyl groups in the substituted compounds [14]. The substitution by a glucuronic acid, a methyl or a sulphate has the same effect: it lowers the charges of all the hydroxyl groups of the flavonoid. Thus, the BDE of all the remaining hydroxyl groups of the metabolites are higher than the corresponding ones in parent compounds. Furthermore, the substitution by an acidic group generally increases the ETE by a large amount. One can partly explain this finding by the fact that protic solvent stabilizes the negative form of the molecule. There are some exceptions, especially acetic acid. For most of the metabolites of quercetin and kaempferol obtained by substitution of one or two hydroxyl group, the gift of an electron has become impossible.
Metabolites resulting from a cleavage of the C-ring: when quercetin is cleaved, two kinds of metabolites are formed: the 3,4-dihydroxyphenylacetic acid and 3,4-dihydroxytoluen preserve the catechol moiety of the B ring. They remain good radical scavengers with BDE of the order of 74 kcal/mole for their different conformations. The substituted toluene has a high DE and remains neutral at physiologic pH. One of the hydroxyl group of 3,4-dihydroxyphenylacetic acid can be cleaved in the colon and the formed 3-hydroxyphenylacetic acid becomes worse radical scavenger by hydrogen atom donation but it keeps very good radical scavenging ability by electron donation. In the tissues, 3,4-dihydroxyphenylacetic acid is transformed in 4-hydroxy-3- methoxyphenylacetic acid. This compound keeps moderate radical scavenging property by donation of hydrogen atom with a BDE of 76.9 kcal/mol but it loses the capacity of donating an electron.
Reference 14 has demonstrated the good agreement between calculated and experimental radical scavenging ability of aglycone and substituted flavonoids. Figure 3 indicates that this agreement continues with metabolites of quercetin since the calculated BDE of 3,4-dihydroxyphenylacetic acid, 3-hydroxyphenylacetic acid and 3,4-dihydroxytoluen display a good correlation with experimental IC50 values measured by DPPH-scavenger assay [25]. The other metabolites issued from the A ring after cleavage of the C-ring, 2,4,6-trihydroxybenzoic acid and phloroglucinol are not good radical scavengers.
Theoretical calculations confirm the decrease of the radical scavenging potency of the majority of metabolites of quercetin or kaempferol. However, the metabolites of quercetin generally remain moderate radical scavengers by donation of a hydrogen atom when they conserve the flavonoid frame. When the C-ring is cleaved, which occurs for the majority of ingested flavonols, some metabolites may retain good radical scavenging properties either by hydrogen atom donation or electron donation in particular when they conserve the B-ring. Other metabolites are deprived from the initial ability. The remaining potency of some metabolites can partly explain the efficiency of diet rich in vegetables in exerting protective effects against many diseases linked to oxidative stress [26]. Perez-Vizcaino et al. [27] have proposed another explanation of the efficiency of diet rich in quercetin. If quercetin aglycone and glycosides are substituted during intestinal absorption and in the liver, some metabolites are certainly deconjugated in the tissues. Indeed, quercetin is not found in human plasma but its presence in tissues has been observed. Shimoi et al. [28] have evidenced hydrolysis of flavonoid glucuronides in human neutriphis and Lee-Hilz et al. [29] in carcinoma cell lines. Thus it seems that in vivo, there is a possibility of deconjugation of the monoglucuronides of quercetin but not of the sulfates. The two possibilities are not exclusive: a part of the ingested flavonoids metabolites, deconjugated or resulting from the cleaving of the A-ring still can act as radical scavengers.
The calculations have been made with an SGI computer and a cluster IBM P5-575 purchased with the funds of the Région Aquitaine, France. The author thanks S. Bercion of the Université des Antilles Guyane for very helpful discussion.