e-ISSN: 2320-0812
e-ISSN: 2320-0812
Mingjin Xun2*, Zhong Feng1,2,3, Yajie Hao2,3, Qingyan Cui2,3, Liying Zhai2,3, Hui Li2,3, Guimin Zhang2,3
1Department of Pharmaceutical Science, Sun Yat-sen University, Shenzhen, China
2Department of Pharmaceutical Science, China Pharmaceutical University, Nanjing, China
3Department of Pharmaceutical Engineering, Guangdong Pharmaceutical University, Guangzhou, China
Received: 23-Dec-2022, Manuscript No. JPA-22-84576; Editor assigned: 26-Dec-2022, PreQC No. JPA-22-84576 (PQ); Reviewed: 09-Jan-2023, QC No. JPA-22-84576; Revised: 23-Feb-2023, Manuscript No. JPA-22-84576 (R); Published: 03-Mar-2023, DOI: 10.4172/2320-0812.12.1.001
Citation: Xun M, et al. In Vitro and In Vivo Evaluation of Whole and Half Tablet of Metoprolol Sustained Release Tablets. RRJ Pharm Anal. 2023;12:001.
Copyright: © 2023 Xun M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Research & Reviews: Journal of Pharmaceutical Analysis
Objective: To investigate the dissolution in vitro and pharmacokinetics in vivo of the original product of metoprolol succinate sustained release tablets and the self-made product.
Methods: In whole tablets and half tablets, a similarity factor (f2) method was used, where the dissolution profiles in four dissolution mediums and pharmacokinetic characteristics in two Beagle dogs of the whole metoprolol succinate sustained release tablets were compared with that of half tablets.
Results: The dissolution behavior of the whole tablets in vitro was similar to that of half tablets and there was no significant difference in the pharmacokinetic parameters tmax and t1/2/h (P>0.05). The Area Under the Concentration time curve (AUC) of half tablet is about half of whole tablet.
Conclusion: The in vitro dissolution and in vivo pharmacokinetics of the whole metoprolol succinate sustained release tablets and the half tablets are similar.
Metoprolol succinate sustained release tablets; Original product; Self-made product; Whole tablet; Half tablet; Dissolution; Pharmacokinetics
As a common oral solid dosage form, scored tablets have the advantages of facilitating dosage adjustment and improving medication compliance for the elderly and children. At present, there are few domestic documents about the divisibility of tablets, in which only the uniformity of the tablet and the single medium dissolution rate after division were discussed. Investigation of important indicators such as the release rate in different mediums and the absorption in vivo of the divided tablet are insufficient. In 2013, the US Food and drug administration issued guidelines on tablet scoring, mentioned that for divisible tablets, the dissolution behavior after division should be compared with the whole tablet, in compliance with the regulations [1].
Metoprolol succinate sustained release tablets are selective adrenal blockers, used in the clinical treatment of hypertension, angina pectoris, coronary heart disease and other diseases, with significant effects and few adverse reactions. The Reference Listed Drug (RLD) was Betaloc produced by AstraZeneca in the United Kingdom and was approved for marketing in 1992. There were 4 specifications (25 mg, 50 mg, 100 mg and 200 mg), among which 200 mg specifications are scored tablets. In this research, scored metoprolol succinate sustained-release tablet (200 mg) was used to study the friability, dissolution behaviors as well as other in vitro indicators and in vivo pharmacokinetic characteristics of the whole and half tablet of RLD and the self-made product. In addition, stability and the drug releasing characteristics in vivo and in vitro of them were compared, providing medication guidance for clinical medication [2].
Instruments and reagents
Instruments: Ultimate 3000 high performance liquid chromatograph (American thermo fisher company), finnigan TSQ quantum ultra triple quadrupole mass spectrometer equipped with Electrospray Ionization Source (ESI) and Xcalibur 3.0 workstation (American thermo fisher scientific company), RC12AD series automatic Dissolution tester (Logan); SY-2D friability tester (Logan); TG328A/S electronic balance (Metter).
Materials and reagents
RLD of Metoprolol succinate release tablet (Lot No: LF0172, trade name: Betaloc, specification 200 mg, British AstraZeneca company), metoprolol reference substance (Shanghai Ruiqi Biological reagent Co, Ltd, content ≥ 98.0%, Lot No: B09S7Q20187), propranolol reference substance (Beijing Biolab technology Co, Ltd, content 99.8%, lot no: 061227), phosphoric acid (analytical pure), sodium dihydrogen phosphate (analytical pure), potassium dihydrogen phosphate (analytical grade), acetonitrile (chromatographic grade), 95% ethanol, sodium lauryl sulfate (chromatographic grade), methanol (chromatographic grade), purified water [3].
Animals: 8 healthy Beagle dogs, ordinary grade, 7.0-9.5 kg, provided by Beijing Max biotechnology Co. Ltd, experimental animal production license number SCXK 2017-0007.
Methods
Segmentation of the tablet: Divide tablets of the RLD and self-made product into 2 halves manually by breaking along the middle score to investigate the changes in weight loss, friability caused by division, release degree and so on. Detection of weight loss: Select 15 tablets of RLD and 15 tablets of self-made product randomly, weigh the mass of each single tablet (Wn) precisely and record. Divide 30 tablets separately by hand breaking, discard chips of the tablets, weigh the mass of two halves of the 30 divided tablets, denoted as Wn-1 and Wn-2, and record. Weight loss was calculated through the equation below and results were shown in Table 1.
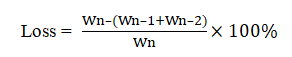
Friability: The dividing surface of RLD that divided manually was not flat. Thus, the product might be worn and broken due to vibration and collision during transitions, which might cause product weight loss and affect the function. Therefore, it was necessary to check the friability of the halves divided by hand breaking method.
According to the friability measurement method proposed in USP1216, 7.0 g tablet sample was suggested. Accurately weigh the whole tablet and the half tablet, each about 7.0 g, discard the powder and place it in the drum of friability tester, rotate the drum at a speed of 25 r/min for 4 min, remove all loose dust from the tablets, accurately weigh them, calculate the friability of the whole tablet and the hand-broken half tablet [4].
Determination method for release
Chromatographic conditions: Column, Agilent Zorbax SB-C8; 4.6 mm × 250 mm 5 μm. DAD detector, wavelength 220 nm. Mobile phase, acetonitrile-0.12% sodium lauryl sulfate solution (60:40, v/v). Column temperature 37.0°C. Flow rate, 1.5 mL/min. The injection volume was 5 μL.
Solution preparation
Reference solution: Accurately weigh about 20 mg of metoprolol succinate reference substance, place it in a 50 mL volumetric flask, dissolve it with dissolution medium and dilute to volume, mix it.
Test solution: Put 6 whole tablets of RLD into 4 dissolution mediums respectively, where the rotating speed are of 50 r/min. 6 half tablets of RLD, 6 whole tablets of self-made product and 6 half tablets of self-made product are disposed equally. Take samples at 1 h, 2 h, 4 h, 6 h, 8 h, 10 h, 12 h, 14 h, 16 h, 18 h, 20 h, 22 h, and 24 h respectively, in a volume of 10 mL. Filter the samples and test them at the chromatographic conditions described in chromatographic conditions [5].
Pharmacokinetic studies
Methods of administration and blood sample collection: A single dose and two period cross over test protocol was used, where 8 male beagle dogs were divided into two groups A and B randomly, 4 dogs in each group, fasting for at least 12 hours before the test. Beagle dogs in group A took the whole tablet of metoprolol succinate sustained release tablets, and the beagle dog in group B took half tablet of metoprolol succinate sustained release tablets. During the weekly washout period, cross dosing the beagle dog. 2 mL of blood samples was collected from the forelimb or hind limb vein of the beagle after administration at 0.5 h, 1 h, 2 h, 2.5 h, 3 h, 3.5 h, 4 h, 6 h, 9 h, 12 h, 24 h, 48 h, respectively, and stored with 1% heparin for anticoagulation [6]. Then all blood samples were centrifuged at 3000 rpm for 10 min, and supernatants were taken and stored in a refrigerator at -20°C for testing.
Methods for determination of plasma samples
Chromatographic and mass spectrometry conditions: Chromatographic conditions were same with that in chromatographic conditions. Mass spectrometry conditions were as follows. Ion source was Electrospray Ionization Source (ESI), whose temperature was 350°C. Atomizing gas pressure was 275.79 kPa, drying gas flow rate was of 10.0 L/min. Monitoring ion pair of metoprolol was m/z 268.2→m/z 116.2, and propranolol (internal standard) was m/z260.2→m/z116.2. Collision energy of metoprolol was 20 eV and propranolol 22 eV.
Plasma sample processing: Firstly, precisely transfer 1 mL of plasma sample into EP tube by measuring pipets, add 50 μL of propranolol solution with a concentration of 23 μg/mL, add 1 mL of sodium hydroxide solution (1 moL/L), vortex and mix for 30’s. Then, add 5 mL of the dichloromethane ether (2:3) mixture, vortex for 5 min, centrifuge (2665 × g) for 10 min, transfer 5 mL of the supernatant into a centrifuge tube, and blow dry with nitrogen. Finally, dissolve the residue with 100 μL of mobile phase, vortexed for 3 min, centrifuged (2665 × g) for 10 min, and take the supernatant to test [7].
Validation of the method: Transfer 1 mL of blank plasma into a centrifuge tube, add 50 μL of propranolol solution with a concentration of 23 μg/mL, add series of standard solutions to prepare plasma samples containing metoprolol 120 μg/mL, 40 μg/mL, 20 μg/mL, 10 μg/mL, 5 μg/mL, 2.5 μg/mL and 1 μg/mL, separately. They were tested with the method in chromatographic and mass spectrometry conditions [8].
Stability study
Determination methods for related substances
Chromatographic conditions: Column, XTERRA RP18, 4.6 × 150 mm 5 μm. Detection wavelength, 223 nm.
Mobile phase: solution A was methanol-phosphate buffer (40:60), solution B was acetonitrile-phosphate buffer (70:30). Column temperature was 30.0°C. Flow rate was 1.0 mL/min. Injection volume was 10 μL. Gradient elution was carried out as follows Table 1.
| Time (min) | Solution A (%) | Solution B (%) |
|---|---|---|
| 0 | 65 | 35 |
| 20 | 65 | 35 |
| 25 | 45 | 55 |
| 30 | 45 | 55 |
| 35 | 30 | 70 |
| 37 | 65 | 35 |
| 50 | 65 | 35 |
Table 1. Gradient elution conditions for determination of related substances.
Preparation of reference substance stock solution: Accurately weigh 12 mg of metoprolol succinate reference substance, place it in a 100 mL volumetric flask, dissolve it with diluent and dilute to a constant volume, mix it. Transfer 5.0 mL of the mixed solution by pipette into a 50 mL volumetric flask, dilute to the volume, and mix it [9].
Sample solution preparation: Take 20 tablets of the product and grind them to powder, firstly. Then, accurately weigh an appropriate amount (equivalent to 50 mg of metoprolol succinate) and put in a 50 mL volumetric flask, add some diluent to the flask, keep the flask in ultrasonic for 30 min. Next, dilute to the volume, and mix it. Finally, filter the sample with a 0.45 μm filter membrane [10].
Determination method for content
Chromatographic conditions: Column, YMC triart C8, 4 mm × 125 mm, 5 μm. Detection wavelength, 280 nm. Mobile phase, acetonitrile-phosphate buffer (25:75). Column temperature was 25.0°C. Flow rate was 1.0 mL/min. Injection volume was 40 μL.
Preparation of reference substance solution: Accurately weigh 25 mg of metoprolol succinate reference substance and put it in a 50 mL volumetric flask, dissolve it with the mobile phase and dilute to the volume, mix it. Transfer 5.0 mL of the mixed solution into a 50 mL volumetric flask and dilute to volume with the mobile phase, mix it [11-15].
Sample solution preparation: Put 1 tablet of the product into a 200 mL volumetric flask, add 5 mL of purified water to disintegrate the tablet, add 60 mL of 95% ethanol to the flask, stir for 30 min. Then add 40 mL of 0.1 N HCl to the flask and stir for another 30 min, dilute to the volume with 0.1 N HCl and mix. Next, take 10 mL of the mixture into a centrifuge, centrifuge at 4000 rpm for 20 min. Finally, transfer 5.0 mL of the supernatant after centrifugation into a 100 mL volumetric flask, dilute to the volume with mobile phase and mix, filter with 0.45 μm membrane.
Weight loss
The weight loss of the RLD and self-made product caused by hand breaking was 0.3% and not more than 3.0%, which met the requirements of the relevant guidelines for scored tablets (Table 2) [16].
| Segmentation | Wn/mg | Wn–1/mg | Wn–2/mg | Weight loss/% |
|---|---|---|---|---|
| RLD | 692.3 ± 0.52 | 344.7 ± 1.02 | 345.8 ± 1.23 | 0.3 ± 0.2 |
| Self-made product | 691.4 ± 0.42 | 342.5 ± 1.03 | 346.2 ± 1.13 | 0.3 ± 0.2 |
Table 2. Wight loss results(x ± s, n=15).
Friability
During the test, there were no cracks or fragments, and the friability of both whole and half tablet was less than 1.0%, which met the requirements of friability. This test showed that half tablet divided by hand along the score met the requirements of carrying and using. Results are shown in Table 3 and Table 4.
| Sample | Initial weight/g | Final weight/g | Friability/% |
|---|---|---|---|
| Whole tablet | 6.85 | 6.84 | 0.15 |
| Half tablet | 6.98 | 6.96 | 0.29 |
Table 3. Friability results of reference product.
| Sample | Initial weight/g | Final weight/g | Friability/% |
|---|---|---|---|
| Whole tablet | 6.83 | 6.81 | 0.29 |
| Half tablet | 6.97 | 6.94 | 0.45 |
Table 4. Friability results of self-made product.
Test of release
There was not interference for metoprolol succinate in blank excipient solution during the detection. The RSD of the peak areas of the reference solution (in quintuplicate) was not more than 2%. The tailing factor was not more than 2.0. The F value was 98%-102%. The regression equation was A=343.78c-72.53, r=0.9997, concentration of metoprolol succinate ranged from 0.05 to 4.66 g/mL. The test solution and reference solution that have been placed at room temperature for 48 h were injected into chromatographic system and the peak areas with that of freshly prepared samples were compared. Changing rates were 0.15% and 0.43%, respectively, indicating that the solutions were stable within 48 h [17-19].
The release rate of both RLD whole tablet and half tablet in the 4 mediums were shown in Figure 1, and that of the self-made product whole tablet and half tablet were shown in Figure 2. The figures showed that release rates of whole tablet and half tablet after 20 h were all greater than 85%.
When similarity factor (f2) was used to compare similarity of dissolution profiles, two dissolution profiles could be considered similar if f2 values were not less than 50, unless otherwise specified.
In Table 5, the f2 values of release profiles for whole tablet and half tablet in four dissolution medias, both RLD and self-made product, were all greater than 50, suggesting that the release behaviors of whole tablet and half tablet in four dissolution medias were similar. In Table 6, the f2 values of release profiles for RLD whole tablet and three batches of self-made product whole tablet in the four dissolution medias were all greater than 50, indicating that the release behaviors of RLD and self-made product were similar [20].
| Samples | Mediums | |||
|---|---|---|---|---|
| 0.1 NHCl | pH 4.0 Acetic acid buffer | pH 6.8 Phosphate buffer | Water | |
| RLD(f2) | 77 | 75 | 78 | 76 |
| Self-made product(f2) | 78 | 78 | 76 | 77 |
Table 5. f2 values of release profiles for whole and half tablets in different mediums.
| Batch | Mediums | |||
|---|---|---|---|---|
| 0.1 NHCl | pH 4.0 Acetic acid buffer | pH 6.8 Phosphate buffer | Water | |
| 1 | 78 | 75 | 73 | 74 |
| 2 | 77 | 76 | 74 | 73 |
| 3 | 77 | 75 | 72 | 73 |
Table 6. f2 values of release profiles for whole tablet of RLD and self-made product in different mediums.
Pharmacokinetic studies in vivo
Validation of plasma measurement methodology: The regression equation was A=1.0186c+0.2623, R=0.9997, indicating that the method was of good linear relationship between concentrations from 1.0 μg/mL to 120 μg/mL of metoprolol and responses. The mean recoveries of low, medium, and high concentration samples were 96.19%, 95.71% and 94.12%, which showed this method was accurate and sensitive and can be used to accurately determine metoprolol in plasma.
Test results: The average metoprolol concentration-time curves in vivo after oral administration of RLD and self-made product, whole and half tablet, were shown in Figures 3-5.
The plasma concentration at different time points in the subject was processed with DAS 2.0 software, which was shown in Tables 7 and 8.
| Parameters | Whole tablet | Half tablet |
|---|---|---|
| AUC0→24 h/h·g·L-1 | 896.84 ± 23.69 | 463.68 ± 15.08 |
| AUC0→∞/h·g·L-1 | 897.94 ± 46.11 | 461.08 ± 21.78 |
| tmax/h | 3.10 ± 0.47 | 2.80 ± 0.72 |
| cmax/g·L-1 | 98.39 ± 2.16 | 44.32 ± 1.93 |
| t1/2/h | 4.50 ± 0.52 | 4.62 ± 0.30 |
Table 7. Pharmacokinetic parameters of RLD after oral administration in dogs body (n=8).
| Parameters | Whole tablet | Half tablet |
|---|---|---|
| AUC0→24 h/h·g·L-1 | 887.84 ± 21.79 | 453.68 ± 15.18 |
| AUC0→∞/h·g·L-1 | 891.51 ± 43.12 | 452.12 ± 20.57 |
| tmax/h | 3.06 ± 0.43 | 2.60 ± 0.70 |
| cmax/g·L-1 | 96.26 ± 2.19 | 43.22 ± 1.7 |
| t1/2/h | 4.50 ± 0.530 | 4.62 ± 0.40 |
Table 8. Pharmacokinetic parameters of self-made product after oral administration in dogs body (n=8).
The pharmacokinetic parameters of the RLD and self-made product were analyzed using T test. There was no statistical difference between tmax/h and t1/2/h (P>0.05), indicating that there is no significant difference in the release and metabolism of the two preparations in body. AUC of half tablet is about half of the whole tablet.
Stability results
During the medication process, tablets of medicine in a single package decreased and top space in the bottle increased, which might affect the stability of drugs. Therefore, stabilities of the RLD and self-made product packaged according to the commercial packaging specifications were studied, which were stored in 25°C ± 2°C/60% Relative Humidity (RH) ± 5% RH conditions for 90 days. Content, relative substances and water content of the tablets were tested at 45 days and 90 days. Results were listed in Table 9.
| Test items | 0 d | 45 d | 90 d |
|---|---|---|---|
| Assay/% | 99.8 | 99.6 | 99.1 |
| Water/% | 2.13 | 2.42 | 2.65 |
| Total impurities/% | 0.32 | 0.27 | 0.3 |
Table 9. The results of stability test.
In the study of metoprolol succinate sustained release tablets, the mass loss rates of RLD and self-made product after breaking along the score were 0.3%, and the fragility of both whole and half tablets were less than 1%, which met the quality requirements of breaking tablets.
The release rate of RLD and self-made product of metoprolol succinate sustained-release tablets, whole and half tablets, in pH 1.2 hydrochloric acid solutions, pH 4.0 acetic acid buffers, pH 6.8 phosphate buffers and water were all greater than 85% in 20 hours. Therefore, the four dissolution mediums were used to investigate the in vitro release characteristics of whole tablets and half tablets. The f2 values of the whole and half tablet in the four mediums were all greater than 50, indicating that the release rates of the whole tablet and half tablet in above-mentioned solvents were similar.
In the pharmacokinetic study, AUC of the RLD of metoprolol succinate sustained release tablets was 896.84 ± 23.69, similar with the previous study, and half tablet was 463.68 ± 15.08, about one half of the whole tablet. AUC of the self-made product was 887.84 ± 21.79, and half tablet was 453.68 ± 15.18, about one half of the whole tablet. The tmax of half tablet was slightly faster than the whole tablet. Under the same in vivo conditions, differences in tmax might be related to the specific surface area of the sample. The specific surface area of the half tablet was larger than the whole tablet, and the contact area with the gastrointestinal tract was larger, which was beneficial to the release and absorption of drugs. In addition, the stirring intensity of the gastrointestinal tract of the beagle dog was stronger than that of the human body, which might also be the reason for the faster release of half of the tablet in the body.
In the stabilities study of open bottles of tablets that stored for 90 days, both whole and half tablet of the RLD and the self-made product met the quality requirements, indicating that qualities of the whole and half tablets were within the specified limits, which provided basis and guarantee for the rational use of drugs.
In vitro release and in vivo pharmacokinetic processes of the whole and half tablet of metoprolol succinate sustained release tablets were studied in this article, which showed that release rates and pharmacokinetic processes of the whole and the half tablets were similar. That is this preparation can be taken in half a tablet during actual use, which possess a more positive significance for the implementation of individualized administration.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]