ISSN:2321-6212
ISSN:2321-6212
Moamen S Refat1,2* and Abdel Majid A Adam1
1Department of Chemistry, Faculty of Science, Taif University, Al-Haweiah, P.O. Box 888, Zip Code 21974, Taif, Saudi Arabia.
2Department of Chemistry, Faculty of Science, Port Said University, Port Said, Egypt
Received: 10 September 2013 Accepted: 22 October 2013
Visit for more related articles at Research & Reviews: Journal of Material Sciences
The In2O3, Bi2O3 and Sb2O3 oxides were synthesized by a novel reaction of urea with InCl3, BiCl3 and SbCl3 respectively, in an aqueous media at ~ 90 oC. The infrared spectra and microanalysis, CHN, of the solid products resulted indicate that the absence of the bands of urea, but appearing the characteristic bands of oxides. A general mechanism describing the formation of oxides and decomposition of urea are suggested.
In2O3, Bi2O3, Sb2O3 and urea
Urea and their derivatives are known to be the parent compound of a large and interesting class in both organic and inorganic compounds; it is used as a starting material for the synthesis of many applied compounds. The literature revels that urea is forming coordinate bonds with many metal ions at room temperature in aqueous and non aqueous media through its oxygen or nitrogen atoms depending on the type of metal ion used [1-8]. From the chemical viewpoint, the reaction of metal salts with urea at high temperature has recently gained increasing interest [7-14]. The nature of the reaction products depend strongly on the type of metal ions and so the metal salt used. In our previously studies referenced by us [7-14] concerning the reaction of urea with metals such as Co( III), pb(II), Sn(II), Cr(III), Fe(III), Au(III), Sn(IV), V(V) and Mo(IV) at high temperature demonstrate that the types of metal ions beside their anions have a pronounced effect on the nature of the reaction products. The published papers owned by us in this trend of the reaction of urea with different metal salts at elevated temperature lead to discovering a novel method for preparation pbCO3 and CoCO3 [10], lanthanide carbonates [12], limonite, FeO(OH) [9], 2ZnCO3.3Zn(OH)2 [8], and SnOCl2.2H2O [7].
The aim of this publication is to report the synthesis and characterization study of the In2O3, Bi2O3 and Sb2O3 oxides resulted from a novel oxidation reduction reaction between urea with InCl3, BiCl3 and SbCl3, respectively, in an aqueous solution at ~ 90 °C.
All chemicals used throughout this study were Analar or extra pure grade. The white oxides, In2O3, Bi2O3 and Sb2O3 were prepared by mixing equal volumes of aqueous solutions of 0.1M of InCl3, BiCl3 and SbCl3 and 1.0 M of urea. The mixtures were heated on a water bath to approximately 90°C for about ~24 h. The white colored precipitate filtered off, washed several times with bi-distilled water and dried in vacuo over CaCl2. The elemental analysis for the obtained products shows the absence of carbon, hydrogen and nitrogen elements. The percentages of indium, bismuth, and antimony were determined by using thermogravimetric method. The infrared spectra of the reactants and the solid products obtained were recorded from KBr discs using a Bruker FT-IR Spectrophotometer.
The reaction of aqueous solutions of urea with InCl3, BiCl3 and SbCl3, produces clear white colored oxides, In2O3, Bi2O3 and Sb2O3. The formation of these oxides upon the heating of an aqueous mixture of InCl3, BiCl3 and SbCl3, respectively, with urea may be understood as follows:
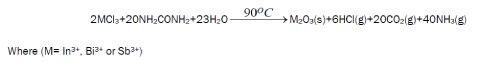
For the reaction mechanisms an oxidation process for In(III), Bi(III) and Sb(III) occurs during the decomposition of urea into ammonia, carbon dioxide and hydrogen chloride gases. The infrared spectra of synthetic oxides products are shown in Fig. 1. The infrared spectra of the obtained products show no bands due to characteristic groups of urea (carbonyl and amide groups), but the bands associated to the oxides are observed [15]. Based on this observation, along with that obtained from elemental analysis data as well as determination of metals In(III), Bi(III) and Sb(III), the fact that infrared spectra of commercially obtained In(III), Bi(III) and Sb(III) are the same as that of the reaction products.
Figure 1: Infrared spectra of the product resulted from the reaction of urea with InCl3, BiCl3 and SbCl3.