e-ISSN: 2320-0812
e-ISSN: 2320-0812
Department of Pharmaceutical Chemistry, JSS College of Pharmacy, JSS Academy of Higher Education& Research, Sri Shivarathreeshwara Nagar, India
Received date: 30/03/2019; Accepted date: 17/04/2019; Published date: 11/05/2019
Visit for more related articles at Research & Reviews: Journal of Pharmaceutical Analysis
Genotoxic impurities, Regulatory documents, Analytical challenges, Limits, Gas chromatography
Recently, genotoxic impurities gained attention from pharmaceutical industry and health authorities as their presence affects the safety, efficacy and quality of the drugs. Regulatory authorities like ICH, EMA, FDA has given the guidelines on identification, control, analytical methods of GTI’s (Genotoxic impurities). Hence these impurities need to be identified, isolated and analysed by different analytical methods to control the GTI’s in active pharmaceutical ingredients. Analysis of GTI’s has become a challenge to pharmaceutical industries which requires selective, sensitive and robust methods. This article provides information on sources, classification, regulatory documents of GTI’s. It also emphasises on risk characterisation, factors for better targeting of analytical methods, analytical challenges, and analytical assessment of genotoxic impurities and genotoxicity prediction. The article mainly describes different techniques of Gas chromatography by which volatile samples can be analysed.
Many of the pharmaceutical drugs are manufactured by chemical synthetic pathway or by modifying the natural product itself. In both the cases, the reagents used and the side products formed in the reaction may occur as impurities in the final drug product. These impurities cause changes in the drug effecting its safety and efficacy which leads to unwanted toxicities in the patient [1]. The major challenge for the pharmaceutical industries is to produce a quality drugs. Hence, many analytical tests are performed to confirm the quality and purity of the final drugs according to the guidelines given by FDA. The analysis of pharmaceutical impurities is a very intensive task involving method development, impurity synthesis, impurity isolation and many analytical procedures to identify the impurity. It involves the identification of structural alerts acting as impurity in a drug substance which are above threshold limits. The chemical structure and the mechanism of how the impurity is formed need to be identified which helps for toxicological assessment, thus improving the synthesis to reduce or prevent the impurities in final drug. Hence, many analytical methods are emerging for quality evaluation of new drugs getting manufactured. According to the Regulations, Impurity profiling needs a high-resolution chromatography technique which is capable of giving reproducibly, separating and detecting the impurities present in pharmaceuticals.
Genotoxic Impurities in Pharmaceuticals
Genotoxic impurities are the compounds which have the potential to damage the cells genetic material (DNA, RNA) at any level of exposure effecting its integrity. Genotoxic impurities in drugs has attracted industry attention due to its extreme negative therapeutic effects on patient health. Any insignificant amount of these impurities can cause genetic mutations, carcinogenicity, and genetic disruption leading to oncological diseases.
Genotoxicity causes interaction with spindle apparatus of cell division leading to aneuploidy, topo-isomerase inhibition, overloading of defense mechanisms, carcinogenesis, mutagenesis, teratogenesis and cytotoxicity. Thus, Control and monitoring of genotoxic impurities is an important task for analysts for development and production of new drugs. Genotoxic impurities may be produced from the starting materials, solvents, catalysts, reagents used in the synthesis of drugs. They cause mutations and increase the risk of Cancers in patients [2].
Organic Pathway
Organic genotoxic impurities are produced through raw materials, intermediates, reagents, solvents, reagents used for synthesis of the drugs or drug substances.
Inorganic Pathway
Inorganic genotoxic impurities may occur due to starting materials, strong acids, strong bases, intermediate catalysts, chemical reagents and traces of filter and through equipment used for production.
Degradation Pathway
Impurities are originated from degradation of drug substance, drug product or microbiological action.
Considering the mutagenic, carcinogenic and resulting control actions, International Council for Harmonisation (ICH) has classified the genotoxic impurities into 5 classes Table 1.
Control limits for genotoxic impurities should be very low than toxic impurities in new drug substances.
M7 (R1)
Assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk [3], provides regulations for identification, characterization, toxicology assessment and control of GTI’s arising through manufacturing or storage.
Guidelines for the control of genotoxic impurities:
• PhRMA position paper: The Pharmaceutical Research and Manufacturers of America, for testing and controlling genotoxic Impurities in pharmaceuticals (2006).
• EMEA: Guideline on the limits of genotoxic impurities. Draft releases for consultation in 2002 and 2004, and final version released in 2006. Regulatory document including concept for the Threshold of Toxicological Concern (TTC).
• EMA, Safety Working Party (SWP): Questions and answers on the Guideline on the limits of genotoxic impurities, published in 2008, 2009, 2010, and 2012.
FDA Guidance for Industry (Draft)
Genotoxic and carcinogenic impurities in drug substances and products: Recommended Approaches (2008), aligned with the EMA guideline [4].
Guidelines for genotoxicity testing:
• ICH S2 (R1): Genotoxicity testing and data interpretation for pharmaceuticals intended for human use. This document combines previous guidelines ICH S2A (1996) and ICH S2B (2007), global document for genotoxicity testing.
• EMA: Guideline on the assessment of genotoxicity of herbal substances/preparations (2008). Guideline describes practical approaches for testing and interpretation of potential genotoxicity of herbal drugs.
European Commission Health & Consumer Protection Directorate
General risk assessment methodologies and approaches for genotoxic and carcinogenic substances (2009).
ICH Q3A(R)-Impurities in new drug substances [5].
ICH Q3B(R)-Impurities in new drug products [6].
ICH Q3C-Guidance for Residual solvents [7].
recommendations (emea guidelines on the limits of genotoxic impurities, 2006)
Q3A guideline states that genotoxic impurities may arise from synthetic pathway, purification process and storage of API which need to be identified contributing to impurity profiling and degradation products of new drug substances. When structural alerts are identified in the API then different in vitro and in vivo tests need to be performed to confirm the genotoxic impurity in API. Q3A guideline recommends that the studies should be performed on the drugs or drug substances containing impurity or by isolating the impurity.
Genotoxic Compounds with Sufficient (Experimental) Evidence for a Threshold-Related Mechanism
Compounds with threshold genotoxicity without any risk can be established according to class 2 solvents. The approach calculates Permitted Daily Exposure (PDE) taken from Lowest Observed effect level (LOEL).
Genotoxic Compounds without Sufficient evidence for a Threshold-Related Mechanism
These compounds have the risk of genotoxicity, hence both pharmaceutical and toxicological assessment need to be done.
Pharmaceutical Assessment
The starting materials, intermediates, synthetic pathway, chemical reagents, need to be assessed for presence of genotoxic impurities. These impurities should be within the limits given by EMEA. If the impurity is unavoidable, then efforts should be made to decrease the impurity in the formulation in compliance with safety and toxicity.
Toxicological Assessment
In the drugs were the genotoxic impurity cannot be avoided, toxicological assessment needs to be done to ensure the toxicity. Here the concept of daily intake limit is implemented for safety of the patient.
Toxicological assessment can be made by in-vivo and in-vitro tests (Ames test, chromosomal aberration test) to set the acceptable limits for genotoxic impurities Figure 1.
Application of threshold of toxicological concern:
The exposure limits for genotoxic impurities are developed by concept of TTC.
Concentration limits for genotoxic impurities in final API can be calculated by considering daily dose.
Concentration limit (ppm)=TTC (μg/day)
As a result of the hazard assessment each impurity will be assigned to one of the 5 classes specified in Table 1. For the impurities belonging to classes 1, 2, 3 the limits are assigned according to Threshold Toxicological Concern (TTC). The EMEA guideline for control of genotoxic impurities were set by using the approach based on Threshold of Toxicological Concern (TTC), as genotoxic impurities cannot be completely removed from drug substance. According to European Medicines Agency’s Committee for Human use (CHMP), Threshold of Toxicological Concern (TTC) of 1.5 μg/day for a lifetime, below which daily intake of genotoxic impurity with unknown carcinogenic or mutagenic potential is unlikely to exceed a lifetime cancer risk of one additional case in a population of 100000 people Tables 2 and 3.
| Class | Definition | Proposed action for control |
|---|---|---|
| Class 1 | Known carcinogens with more risk of genotoxicity | Control at or below compound specific acceptable limits (TTC) |
| Class 2 | Known mutagens with unknown carcinogenic potential | Control at or below specific acceptable limits (appropriate TTC) |
| Class 3 | Drug structural alerts, not related to drug structure No genotoxic, mutagenic data |
Control at or below acceptable limits (TTC) or conduct QSAR studies. Non-mutagenic=Class 5 Mutagenic=Class 2 |
| Class 4 | Alerting structures are same as that of functional groups related to drugs which are tested and found to be non-mutagenic | Non-genotoxic impurity |
| Class 5 | No structural alerts with sufficient data, evidence indicates lack of genotoxicity or carcinogenicity | Non-genotoxic impurity |
Table 1: Classification of genotoxic impurities.
| Duration of activity during Clinical trials | Threshold value of genotoxic impurities, µg/day | ||
|---|---|---|---|
| PhRMA | FDA | EMEA | |
| Single dose | 120 | 120 | 120 |
| <14 days | 120 | 60 | 120 |
| From 14 days – 1 month | 120 | 60 | 60 |
| From 1-3 months | 40 | 20 | 20 |
| From 3-6 months | 20 | 10 | 10 |
| From 6-12 months | 10 | 5 | 5 |
| >12 months | 1.5 | 1.5 | 1.5 |
Table 2: Allowed threshold values established by PhRMA, FDA, EMEA for genotoxic impurities.
| Treatment Duration | Daily Dose µg/day Drug content |
|
|---|---|---|
| One genotoxic impurity | Several genotoxic impurities | |
| ≤ 1 month | 120 | 120 |
| >1-12 months | 20 | 60 |
| >1-10 years | 10 | 30 |
| >10 years or for lifetime | 1.5 | 5 |
Table 3: Allowed doses established by ICH M7 (R1) for genotoxic impurities.
• 1.5 μg/person/day-1:100000 lifetime risk for cancer
(Provided there is an expected over riding benefit of drug)
• 0.15 μg/person/day- 1:1000000 lifetime risk for cancer.
EMEA recommends an adjustment factor for children:
• Ages 0-2: 10-fold exposure level decrease
• Ages 2-16: 3-fold exposure level decrease
General Method Design and Evaluation for Genotoxic Impurities
General analytical method design to determine the genotoxic impurities can be achieved by considering the properties of the analyte, sample matrix, Analytical technique used in unit operation. Generally, for primary GTI analysis, simplest techniques like HPLC using PDA detection (non-volatile drugs) and GC with FID detection (volatile drugs) should be used. When the proposed method doesn’t show desired sensitivity then further other techniques can be employed for the analysis [8]. The decision tree for selecting the accurate Analytical technique was given in the Figure 2. It starts with solution stability of the analyte which determines requirement of alternative sample preparation techniques rather than directly diluting and injecting the analyte into system. Poor stability of the analyte can be increased by storing it at low temperatures or protecting the analyte from light. Chemical methods such as matrix deactivation and derivatization can also be employed. Next to stability, the sample volatility plays a role in choosing GC or LC (Liquid Chromatography) technique. The derivatization methods need to be considered for selecting the detectors to LC or GC. For the designed method to be evaluated it should show good resolution for the analyte peak to that of other interfering peaks. Eventually the method should possess desired sensitivity, linearity, accuracy and precision.
Factors that Ensure Better Targeting of Analytical Method
Durational limits: The control of genotoxic impurities to TTC levels are required during phases of clinical trials. The original guidance is not suitable for this purpose because it is too rigid. Specifically, TTC depends on life time exposure considerations which is 75 years but the exposure in early phase clinical trials is <30 days. So pharmaceutical industry has proposed a staged TTC which developed gradually to LTL limits and are included in current ICH M7 (R1) guidance, applied to both clinical and commercial pharmaceutical drugs.
Permitted daily exposure: The addendum to ICH M7(R1) introduced the concept of Acceptable Intake (AI) or Permitted Daily Exposure (PDE) for genotoxic reagents containing safety data which can be used as a default TTC limits, providing reliability in terms of acceptable limits that can be changed to analytical profile for analytical method i.e. Limit Of Quantification (LOQ).
Control possibilities: According to ICH M7 (R1), these possibilities show a profound influence on the routine analysis and method development for genotoxic impurities.
a) Possibility 1: Test for genotoxic impurity in final Active Pharmaceutical Ingredients.
b) Possibility 2: Test for genotoxic impurity in raw materials, starting materials, intermediates used in the synthetic reaction for their limits in permitted level.
c) Possibility 3: Testing intermediate stage of synthetic reaction which have higher limits understanding the process capacity.
d) Possibility 4: No tests are required as genotoxic impurities in drug are more reactive.
Analytical Challenges
Baker was the first to study the challenges associated with analysis of GTI in final drugs [9]. They include:
1. Low limits: Genotoxic impurities have very low limits which are based on dose and duration of exposure. The default lifetime TTC limit, LOQ/LOD << 1 ppm can be applied in extreme cases of high dose of drug i.e. > 1 gm/day.
2. Matrix interference: During the analysis of Genotoxic impurities by Mass Spectroscopy, the API or intermediates used for analysis act as a interfering matrix and effects the accurate measurements through co elution or ion suppression.
3. Physico-chemical properties of the genotoxic impurity: In some cases, genotoxic impurities are unstable, highly reactive, non-volatile; non-chromophoric which is a challenging task for direct analysis and detection in drugs.
Gas Chromatography
Gas Chromatography is the technique which is used to quantify the volatile GTI in drug substances. Stationary phase is a column which contains microscopic layers of liquid or polymer on inert solid support. Mobile phase is a carrier gas which is helium for determining GTI in drug substances. The injection modes used are direct liquid injection and headspace for GTI analysis. The concentration limits calculated based on daily dose of API plays a key role in determining the desired sensitivity of the method and subsequently selecting detector for the analysis Figure 3.
Analyte Extraction Methods
In cases where GTI and matrix have diverse chemical properties, the extraction methods such as Solid Phase Extraction (SPE), Solid Phase Micro Extraction (SPME), Liquid Liquid Extraction (LLE), Liquid Phase Micro Extraction (LPME), helps to enhance the sensitivity and analyte concentration by reducing matrix interference. Small neutral molecules like alkylating agents show high solubility in organic solvents like hexane and can be extracted out from ionizable drug matrix. Hexane is the most widely used best organic solvent for liquid liquid extraction as only scattered API or impurities can be extracted from the matrix. The solvent does not affect the selectivity or sensitivity of the analytical method [10]. However liquid liquid extraction method is more laborious and may be prone to interferences as it is tough to break the emulsions and many concentration steps involved. To compensate the loss of analyte during the extraction process, additional internal standard need to be used. Solid phase micro extraction is a solvent free extraction method. This method uses coated fibres as a solid phase for extraction of various analytes in liquid (direct injection) or gas (headspace) phase [11]. pH of the coated fibres (solid phase) plays a key role in ionization and extraction of potential interference analytes. pH of the coated fibres should range from 4-9. For ultra-trace analysis of various analytes like alkyl halides and aromatic compounds, Polymer Ionic Liquids (PIL) with different chemical properties are used as a coating fibre (solid phase) for extraction process [12].
GC Detection
GC detection methods like GC-FID, GC-ECD does not show the accurate sensitivity and selectivity to control or monitor the genotoxic impurities in low ppm i.e. below PDE level which is given ICH M7 R(1). The sensitivities of GC-MS and GC-ECD methods for the determination of ethyl chloride were assessed by Liu et al. To overcome this, Mass detection using Single Ion Monitoring (SIM) is used for GTI analysis. However, the low volatility and high thermal stability of ionic liquids allows them to be used at higher headspace oven temperatures with minimum matrix interference leading to increase in the analyte response. E.g. Nitro aromatics use classical Electron Capture Detection (ECD) for quantifying the impurities in ppm levels [13].
Typically, hyphenated techniques i.e. GC-MS or GC-MS/MS with Electron Ionisation (EI), Chemical Ionisation (CI) facilitated by SIM or SRM modes are used for GTI analysis.
These are versatile, sensitive and selective analytical approaches for analysis of genotoxic impurities [14].
GTI Analysis by Headspace GC (HS-GC)
Headspace gas chromatography is widely used analytical technique if the analyte is volatile and allowing it to be present in headspace at high concentration, reducing the matrix interference by dissolving it in high boiling point solvent. Different HS techniques used for analysis includes, Static Headspace Sampling (SHS), Dynamic Headspace Sampling (DHS), Headspace-Solid Phase Micro Extraction (HS-SPME). Typical sensitivity of the techniques is as follows: SHS<SPME<DHS. In recent approaches Ionic liquids are used as versatile diluents in contemporary to DMSO (Dimethyl Sulfoxide), DMF (Dimethyl Formfamide) and DMAC (Dimethyl Acetamide) etc. The use of ionic liquids increases the application of HS-GC analysis for analytes with higher boiling points (≥ 130◦C). These Ionic liquids are chemically inert, exhibit low vapour pressures and possess high stability at temperatures above 350◦C which are ideal physicochemical properties required for optimised headspace conditions [13].
Static Headspace Sampling (SHS) is widely used technique for analysis of genotoxic impurities in pharmaceutical industry. In SHS, only the gaseous components are introduced into sample vial minimising other non-volatile matrix components resulting in high sensitivity of the method. The suitable low temperature programme is developed and optimised for partitioning of the analyte from sample matrix. Recently, studies on trace level analysis of high boiling Genotoxic impurities by HS-GC/ECD namely alkyl/aryl halides nitro aromatics are reported.
GC Derivatisation Technique
Headspace Gas Chromatography techniques can be used for analysis of non-volatile analytes which are derivatised to volatile analytes by different derivatisation reactions. Sample derivatisation is a chemical modification of analyte’s physicochemical properties, increasing the analyte stability, ionisation efficiency and volatility required for mass detection.
This derivatisation method is used for analysis of various genotoxic impurities which include sulfonate esters, hydrazine, alkylating agents etc [15].
Sulfonate esters are the reactive genotoxic impurities formed by the chemical synthesis reactions with residual solvents which causes drug instability Table 4.
| Derivatizing agent | Detector | Analyte | Quantification limit | Reference |
|---|---|---|---|---|
| Methanol | MS | Benzoyl chloride | 0.2 ppm | Raman [18] |
| BSTFA | MS | 4-Chloro-1- Butanol | 0.05 ppm | Harigaya [19] |
| Sodium thiocyanate | FID MS (EI) | Mesylates, DMS | 5-10 ppm ≤0.05 ppm | Lee [20] |
| Benzaldehyde | ECD | Hydrazine | <1 ppm | Carlin [21] |
Table 4: Literature references for application of GC derivatization analysis
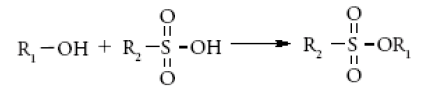
Sulfonate esters lack required vapour pressure for their analysis by HS-GC. Due to the ester hydrolysis, API decomposition and matrix interferences, analysis of sulfonate esters by direct injection method is not possible.
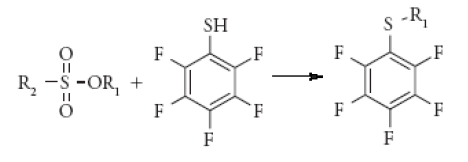
Hence, sulfonate esters are derivatized using pentafluorothiophenol reagent. In this reaction sulfonate esters are derivatised to form sulphides which are volatile in nature [16].
Hydrazine, a degradation product of Isoniazid (anti tuberculosis drug) is a genotoxic impurity which shows high polarity, less volatile and absence of chromophore which is a challenging task for GTI analysis.
Hydrazine is derivatised with benzaldehyde which gives benzalazine, thus making the analysis simpler using GC.
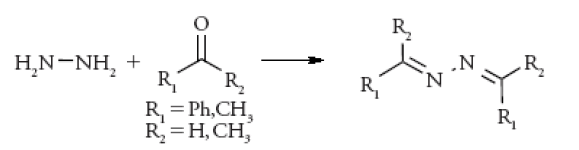
When the derivatised sample is kept intact for longer period before analysis, it has shown increase in derivative (benzalazine). Hence, derivatisation time of a sample plays a critical role. To compensate this, other derivatising agents like acetone are used. Here hydrazine is reacted with acetone (a diluent and derivatising agent) which forms acetone azine. The obtained derivative is further analysed by GC-MS [17].
GC-Direct Analysis Method
GC using direct liquid injection is the primary technique for analysis of volatile and thermally stable analytes-alkyl mesylates which lack vapour pressure required for headspace analysis even after derivatization [22]. Dissolve and Inject approach–dissolving very concentrated solution of drug in non-volatile solvents i.e. DMSO, DMF etc. Because of easy availability and versatility, Flame Ionisation Detector can be used for high sample loading of low dosage drugs which have high safety limits (ICH M7, 2014). However, sensitivity is still often limited. Determination of epichlorohydrin in API using GC-MS in SIM mode with high sample loading (45 mg/mL) with good sensitivity (LOQ 0.85 ppm), accuracy (97.2%), and precision (1.2% RSD) at designated TTC (8 ppm) was reported [23].
To provide a better desired sensitivity and selectivity for GTI at trace levels, a hyphenated GC-MS (SIM)-Selected Ion Monitoring can be employed. Mesylates and alkyl halides can be analysed by using this method were the prominent fragment ion is selected for obtaining the desired sensitivity and resolution [24] because of the accumulation of drug and matrix interferences, the injection volume is restricted to 1 μL which requires frequent changing of inlet. As most of the drugs are non-volatile in nature, higher concentrations are required to achieve the desired sensitivity. During method validation this inlet contamination may produce irreproducibility and less recovery issues.
Enhancing Method sensitivity by removing matrix interference: Presence of excess matrix interferences can produce significant negative impact on desired sensitivity and carryover of the method due to GC detector inlet contamination. For complex analyte samples, matrix deactivation analyte extraction, and 2 Dimensional (2D-GC) can be used rather than introducing nonvolatile and reactive materials. These techniques are used for enrichment of analytes, for selective extraction and to enhance the analyte stability Table 5.
| Sample Preparation Technique | Analyte | Detection mode | Quantification limit | Reference |
|---|---|---|---|---|
| Headspace GC | Alkyl Chlorides | FID | 0.8 (13.5 ppm) | Kaleemullah, 2011 [25] |
| Matrix Deactivation | Various analytes | MS | <1 ppm | Sun, 2010 [26] |
| SPME | Sulfonates | MS(SIM) | 5 ppm | Colon, 2005 [27] |
| LLE direct injection | DMS | MS(SIM) | 0.3 (1 ppm) | Zheng, 2009 [28] |
| LLE direct injection | Mesylates, besylates | MS | <0.1 ppm | Wollein, 2012 [10] |
| 2D-GC | Multiple analytes | MS | <1 ppm | Frank, 2010 [29] |
Table 5: Literature References for application of GC Direct Analysis.
2D-GC Technique
2D-GC is a multidimensional gas chromatography technique which utilises two different columns with two different stationary phases. In this technique effluent from the first column is switched to second column through a modulator. Compounds (epoxides, Michael reaction acceptors) that lack volatility for headspace can be analysed by this technique. This technique equipped with Dean switching valve and MS detector can be used in diverting the matrix to waste and the analyte with desired GTI to mass detector. The second column temperature can be optimized by primary column which results in good selectivity and sensitivity of the target genotoxic impurities from matrix interferences. API, solvents and derivatising agents are not introduced to the seconddimension column and to detector which reduces their overloading and contamination. However, 2D-GC results in complete separation of the multiple impurities present in a sample with high sensitivity and reproducibility. Hence, it is an effective tool for the genotoxic impurity profiling [29].
The following structural alerts present in drugs are noticed to cause genotoxicity. Hence these are reported as genotoxic impurities which should be removed or in cases where it is unavoidable, these should be within limits to prevent the toxicity to the patient [30].
Alkyl esters of phosphoric acids and sulfonic acids
Aromatic nitro, azo-groups
Aromatic ring N-oxides, mono & di-alkyl amino groups
Alkyl hydrazines, alkyl aldehydes
N-methylol derivatives, N-hydroxy derivatives
Monohaloalkanes
Propiolactones & propiosulfones
Aromatic & aliphatic aziridinyl derivatives
Halogenated methanes
Urethane derivatives (carbamates)
Monohaloalkanes
Aliphatic epoxides, nitro groups & aromatic oxides
Viracept (Nelfinavir), Anti-HIV drug by Roche was recalled due to its contamination with a genotoxic substance.
The concept of predicting, extent of genotoxicity from the structural alerts in pharmaceuticals in well established. Structural alerts are the molecular functionalities which are responsible to cause genetic mutations. Regardless of any assumptions, all the impurities with these structural alerts need to be subjected to TTC limit. From the synthesis to manufacture of the drug, it is highly difficult to determine the genotoxicity potential in pharmaceuticals. Hence, the confirmatory tests are done only for the intermediates, compounds which are identified as genotoxic hazards by in-silico methods. In-silico testing methods are used to predict the potential of genotoxicity in new compounds based ion QSAR (Quantitative Structure Activity Relationship) studies.
Ashby and Tennant (1991) reported that about 300 chemicals have shown some correlations of electrophilicity with DNA reactivity (assessed by Ames-testing data) and elucidated the concept of structural alerts for genotoxic activity in the 1980s/1990s [31].
Correlation between 44 substructures and bacterial mutagenicity was established using a database of >4000 compound.
A high proportion of genotoxic compounds were found in epoxides (63%), aromatic nitro compounds (49%), and primary alkyl monohalides (46%). Aromatic amines, aromatic nitros, alkylating agents and Michael acceptors were found to be most affecting structural alerts present in starting materials and intermediates [32].
QSAR studies mainly uses high experimental data and contribute to know the practical aspects to determine the activity of the new untested chemicals based on their structure. As error (e.g. incorrect molecular structure or erroneous data from toxicology studies of a chemical) is introduced into the model, amplification of that error is generated and represented in the prediction.
Carcinogenic potency database consists of 627 chemicals tested in mice for genotoxicity and was reported as TD50 values. Non genotoxic chemicals and mechanisms of carcinogenesis were listed in MULTICASE software. The prediction capabilities of the system for identifying carcinogens and non-carcinogens were 70% and 78% for a modified validation set.
Tafazoli et al. (1998) used the Micronucleus (MN) test and the alkaline single cell gel electrophoresis (Comet) assay for analysing potential genotoxicity of five chlorinated hydrocarbons. Using this data, QSAR studies were done which have shown that LBC_C1 (longest carbon-chlorine bond length), MR (Molar Refractivity), and ELUM0 (Energy of the Lowest Unoccupied Molecular Orbital, indicating electrophilicity) are the factors to be considered for differentiating the genotoxic and non-genotoxic substances [33].
Benigni et al. (2005) showed that the QSAR models are used to predict the genotoxicity of simple unsaturated aldehydes based on their chemical structure. It is commonly accepted that the carcinogenicity of chemicals is owing to their genotoxicity and the mutation and carcinogenesis data are practically coincident. Thus, the two endpoints were added into one ‘‘genotoxicity’’ classification, in which QSAR analysis was applied [34].
QSARs cannot be applied to models for organometallics, complex mixtures (e.g. herbal extracts), and high molecular weight compounds such as polymers [35]. However, the QSAR predictive software offers a rapid, reliable, and cost effective method of identifying the potential risk of chemicals that are well represented in QSAR training data sets, even when experimental data are limited or lacking.
These models should be further developed/validated by employing new mechanistic findings and using newly reported experimental data [36].
Genotoxic impurity profiling in API & Pharmaceutical compounds plays a vital role in clinical development. Hence, a specific, accurate and robust analytical method need to be employed for detection and control of these genotoxic impurities below TTC level. The choice of method need to be decided by considering all the physicochemical properties of the analyte for detection as a method development strategy. Gas Chromatography techniques are used when the analyte is volatile in nature. Considering the sensitivity requirements, the literature reflects the use of hyphenated techniques i.e. GCMS/MS for GTI analysis. Other techniques such as HPLC/MS, CE, HILIC, NMR, and SFC can also be used for GTI analysis. Thus, the selectivity achieved by CE, SFC and HILIC are complimentary to GC and can be used in confirming the genotoxic impurity or to address matrix interferences.
Control strategies for genotoxic impurities are focused on safety limits given in ICH M7 (R1). By considering the regulatory control strategies the highly sophisticated, sensitive, hyphenated analytical methods are developed. With advancements in MS detection, the hyphenated GC-MS/MS techniques are widely used for GTI analysis.
The author does not have a commercial or other association that might pose a conflict of interest with any organization.