E- ISSN: 2320 - 3528
P- ISSN: 2347 - 2286
E- ISSN: 2320 - 3528
P- ISSN: 2347 - 2286
1University of the Punjab, Lahore, Pakistan
2University of the Sargodha, Pakistan
Received date: 12/12/2016; Accepted date: 24/03/2017; Published date: 30/03/2017
Visit for more related articles at Research & Reviews: Journal of Microbiology and Biotechnology
Alternaria leaf spot disease is an important disorder causing serious yield loss of Brassica spp. The current study was designed to evaluate the role of rhizobacteria in management of Alternaria leaf spot disease in Brassica campestris. For this purpose B. campestris seedlings were treated with fifteen rhizobacterial strains under greenhouse and field conditions. The effect of rhizobacteria on quantity of defence related biochemicals such as PAL, PPO, PO and total phenols was examined. The Bacillus megaterium ZMR-6 and Pseudomonas fluorescens RB4 treated plants were least affected by the pathogen under greenhouse condition. Moreover, B. megaterium ZMR-6 and P. fluorescens RB4 exhibited significantly higher amount of defence related biochemicals. The growth promoting traits of bacteria B. megaterium ZMR-6 and P. fluorescens RB4 showed these were capable to exhibit phosphate solubilization, siderophore production and auxin synthesis. The B. megaterium ZMR-6 exhibited ACCD activity but P. fluorescens RB4 devoid this character. The application of peat moss based carrier material of B. megaterium ZMR-6 and P. fluorescens RB4 under field conditions showed promising results for disease management and agro-economic aspects of B. campestris. The current research revealed that B. megaterium ZMR-6 and P. fluorescens RB4 induce systemic resistance against Alternaria leaf spot and enhance yield in B. campestris.
Mustard is an important oil seed crop which fulfills about nineteen percent oil requirement of the world [1]. Alternaria leaf spots caused by Alternaria brassicae is one of the most destructive disease of this crop which cause 47% yield loss [2]. A. brassicae infect foliage and pods of the brassica crop resulting in lesion formation on leaves along with shattering of pods [3].
The disease causing pathogens reduce yield of the edible agronomic and horticultural crops over 10% [4]. A. brassicae is a soil borne cosmopolitan fungal pathogen which causes brown spot or Alternaria leaf spot in different crops [5,6]. Most of the crop cultivars are susceptible to this pathogen due to which proper management becomes a tricky task [7]. On the other hand, fungicides cause environmental issues and become ineffective because fungus may develop acquired resistance against these chemicals. These facts necessitate application of long lasting, economical and eco-friendly techniques for disease management [8]. The research regarding use of root colonizing bacteria as bio-control agents has gained popularity in recent era. The nonpathogenic rhizobacteria may be used as a substitute for chemicals to improve plant growth and resistance against different pathogens [9]. The metabolic activities of this plant growth promoting rhizobacteria (PGPR) including synthesis of growth hormones, siderophore production and phosphate solubilization improve growth and vigor [10-13]. Similarly, the PGPR with ACCD activity have an additional advantage of reducing production of ethylene and reactive oxygen species in plants under stress [14]. Hence, PGPR with ACCD activity help plants to ameliorate the detrimental effect of biotic and abiotic stress [15,16]. These PGPR improve metabolic activities resulting in increased root growth and subsequent nutrients uptake in associated plants [17]. Some rhizobacteria also produce antimicrobial substances such as lactic acid, exotoxins, bacteriocins, antibiotics and lyso-zymes [18]. Researchers have found that non-pathogenic entities may stimulate defense system of plants prior to invasion of disease causing pathogen [19,20]. PGPR also generate antagonistic chemicals which decline injurious effects of pathogens and improve plant resistance against disease causing agents [21]. This plant defense mechanism is known as induced systemic resistance (ISR) and is very effective disease managing strategy [22]. PGPR may induce systemic resistance in associated plants by triggering pathogenesis related (PR) genes. PR genes are involved in activation of defense related enzymes such as peroxidase (PO), polyphenol oxidase (PPO) and phenylalanine-ammonia-lyase (PAL). The phenolic compounds which are synthesized by PPO also play a pivotal role in development of plant resistance.
The aim of present study was to screen rhizobacteria which may manage Alternaria leaf spot in pepper and to elucidate the disease managing strategy.
Evaluation of Bio-Control Efficacy of Rhizobacteria
The rhizobacteria and virulent strain of A. brassicae used during current research were obtained from University of the Punjab, Lahore, Pakistan (Table 1). The rhizobacteria inoculum (104 cfu/mL) was obtained by taking OD of 0.1 at 600 nm. Pathogen inoculum was prepared by harvesting both micro- and macro-conidia from seven days old cultures grown on sterile PDA media at concentration of 1 x 103 conidia/ml, by haemocytometer.
| Bacterial spp. | Disease Index (%) | Control Effect (%) |
|---|---|---|
| Acinetobacter sp. 334 | 22.45 ± 1.58bc | 13.53 ± 1.53f |
| Acinetobacter sp. CS9 | 18.01 ± 2.31c-e | 24.73 ± 3.83e |
| Aminobacter aminovorans 374 | 23.17 ± 3.46bc | 10.46 ± 2.93fg |
| Bacillus fortis 162 | 18.16 ± 2.86d-f | 28.21 ± 2.10de |
| Bacillus megaterium ZMR-6 | 15.82 ± 2.26f | 46.14 ± 3.37bc |
| Bacillus subtilis 170 | 16.41 ± 3.92ef | 35.51 ± 3.62bc |
| Bacillus subtilis 189 | 17.00 ± 2.60ef | 31.42 ± 2.91cd |
| Bacillus thuringiensis 199 | 17.26 ± 0.96ef | 32.37 ± 2.73cd |
| Bordetella pertussis 263 | 20.74 ± 1.76bc | 17.25 ± 1.16ef |
| Burkholderia capacia 337 | 21.87 ± 1.75b-d | 12.32 ± 1.53f |
| Burkholderia cepacia CS8 | 18.34 ± 3.67d-f | 29.15 ± 2.44de |
| Enterobacter sp. CS2 | 22.41 ± 2.26b-d | 13.23 ± 2.72f |
| Microbacterium lacticum 261 | 25.30 ± 3.43b | 03.71 ± 0.57h |
| Pseudomonas fluorescens 083 | 25.34 ± 1.08b | 06.58 ± 1.05gh |
| Pseudomonas fluorescens RB4 | 13.83 ± 3.05g | 49.36 ± 4.64a |
| Sterilized distilled water | 56.64 ± 4.23a | - |
Table 1: Potential of Bacterial spp. to control Alternaria leaf spot disease in Brassica campestris; Values are mean ± standard deviation (n=3). Different letters represent significant difference at (P ≤ 0.05) according to ANOVA and DNMRT.
The pre-sterilized brassica seeds were sown in sterilized loamy soil placed in plastic pots. The soil of 30 days old seedlings was inoculated with 50 ml bacterial inoculum by soil drench method. Next day seedlings were sprayed with pathogen inoculum with the help of hand spray. The distilled sterilized water was used for control treatments. The effect of disease rating scale and subsequent disease index and control effects were analyzed after 25 days of inoculation [23,24].
The bio-control effect (%) was determined as under:
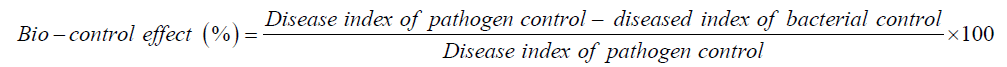
Preparation of Plant Extract
The prewashed plant samples (2 g) were frozen by using liquid nitrogen and ground to powder form with the help of mortar and pestle. The ground samples were re-suspended in cold phosphate buffer (0.05 M) containing polyvinylpolypyrrolidone (0.5 g) at pH 7.0 (1:5 plant tissues to buffer ratio). After homogenizing this mixture by vortex mixture, the homogenate was centrifuged at 14,000 × g at 4°C for 20 min [25]. Subsequently the supernatant were used for evaluation of PO, PPO and PAL activity.
Analysis of PO Activity
PO activity was determined by measuring the guaiacol oxidation in the presence of hydrogen peroxide (H2O2) as described by Fecht-Christoffers et al. [26]. For evaluation of PO activity, 100 μl crude plant extract was mixd with 3 ml solution containing 1 ml each of 0.25% guaiacol H2O2 (0.1 M) and phosphate buffer (0.01 M). The PO activity was measured colorometrically with the help of spectrophotometer by taking absorbance at 470 nm at an interval of 2 min. The solution devoid of plant extract served as a blank [27].
Analysis of PPO Activity
The plant extract (100 μl) was mixed with 0.1 M borate buffer (1.15 ml) at pH 8.8 and 10 mM L-phenylalanine (1 ml). The homogenate was placed in water bath at 40°C for 60 min. The reaction was stopped by adding 250 μl of hydrochloric acid (5 N). The trans-cinnamic acid generated from L-phenylalanine was quantified spectrophotometerically at 290 nm to evaluate PAL activity [27].
Estimation of Total Phenolics
Total phenolics were quantified according to Zieslin and Ben-Zaken [28]. For this purpose, 1 g plant sample was extracted at 70°C with 80% methanol (10 ml) for 15 min. Then 1 ml of this extract was homogenized with 5 ml distilled water and 1 N Folin-Ciocalteau reagent (250 μL) at room temperature. By taking gallic acid as blank, the quantity of phenolics in mixture was estimated spectrophotometrically at 725 nm.
Evaluation of Growth Promoting Attributes of Screened Rhizobacteria
The ACCD activity of screened rhizobacteria was analyzed according to Penrose and Glick [29]. While, phosphate solubilization, siderophore production and auxin synthesis potential of screened bacteria was analyzed according to Mehta and Nautiyal, Schwyn and Neilands and Nautiyal [30-32] respectively.
Evaluation of Screened Rhizobacteria Under Field Conditions
For field trial, split plot design having 5 replicates for each treatment was used. The experimental plot was divided into beds having 3 x 2 m2 sizes. After priming brassica seeds with bacterial inoculum, these were sown and pathogen was applied after 25 days of sowing. The data regarding plant growth attributes was evaluated after 90 days of sowing.
Preparation of Peat Moss Based Inoculum of Bacterial Inducers
The peat moss was sterilized with the help of autoclave and inoculated with inoculum of screened rhizobacteris. For single bacterial inoculation, 100 g sterilized peat moss was inoculated with 50 ml of bacterial. For mixed bacterial formulation 100 gm sterilized peat moss was inoculated with 25 ml of both bacterial inoculums.
Statistical Analysis
The differences between the values obtained were estimated by performing one-way analysis of ANOVA and DNMRT at a significance level of 0.05 in DSASTAT software. Each trial was conducted for 5 biological repeats and the values represented in table’s exhibit the average values of 5 replicates.
Potential of Bacterial Spp. against Alternaria Leaf Spot Disease of B. Campestris
The aim of current study was to screen some native bacterial spp. having potential to induce systemic resistance in B. campestris against Alternaria leaf spot caused by A. brassicae. In case of greenhouse experiment, typical symptoms of Alternaria leaf spot were observed on pathogen challenged plants. In general, the rhizobacteria inoculated plants challenged with pathogen showed delay in leaf spot symptoms, to some extent, as compared with pathogen control plants. However, B. megaterium ZMR- 6 and P. fluorescens RB4 inoculated plants exhibited reduced disease index and better control effect (Table 1). Therefore, B. megaterium ZMR-6 and P. fluorescens RB4 were screened for downstream experimentation.
Elucidation of Biochemical Basis of Induced Resistance
During present study, inoculation of bacterial spp. helped B. campestris plants to synthesize significantly higher quantity of phenolics, PPO, PAL and PO in contrast to pathogen control (Table 2). It was observed that B. megaterium ZMR-6 and P. fluorescens RB4 enhanced phenolics level up to 62.28% and 49.16% respectively as compared with sterilized water control. Similarly, an improvement of 38.45%, 52.83% and 49.95% was observed in PPO, PAL and PO levels by inoculation of B. megaterium ZMR-6 as compared to sterilized water control.
| Bacterial spp. | Phenolics (µg/h/gfw) | % IOUC | PPO Activity ( µg/h/gfw) |
%IOUC | PO Activity ( µg/h/gfw) |
%IOUC | PAL Activity (µg/h/gfw) |
% IOUC |
|---|---|---|---|---|---|---|---|---|
| Acinetobacter sp. 334 | 1.51 ± 0.06f-h | 16.51 ± 2.67j | 5.46 ± 0.51e-g | 25.67 ± 1.52f | 1.05 ± 0.15e-g | 19.61 ± 1.19g | 2.01 ± 0.39bc | 04.37 ± 0.82fg |
| Acinetobacter sp. CS9 | 1.76 ± 0.07d-f | 32.76 ± 2.25fg | 5.92 ± 0.59bc | 32.48 ± 2.07c-e | 1.23 ± 0.08cd | 35.21 ± 2.53d | 2.09 ± 0.64bc | 16.04 ± 2.46e |
| Aminobacter aminovorans 374 | 1.73 ± 0.09d-f | 30.87 ± 1.91gh | 5.54 ± 0.62e-g | 35.55 ± 4.48e | 1.21 ± 0.18d | 30.98 ± 2.18e | 2.05 ± 0.42bc | 07.29 ± 1.04f |
| Bacillus fortis 162 | 1.82 ± 0.10c-e | 39.14 ± 2.92e | 5.42 ± 0.37f-i | 24.53 ± 2.43f | 1.22 ± 0.13d | 30.78 ± 2.51e | 2.14 ± 0.08b | 12.45 ± 2.03e |
| Bacillus megaterium ZMR-6 | 2.14 ± 0.09c | 53.47 ± 4.52c | 6.89 ± 0.72ab | 39.56 ± 3.55c-e | 1.41 ± 0.12a | 52.70 ± 3.44a | 3.01 ± 0.36a | 54.87 ± 4.63b |
| Bacillus subtilis 170 | 2.06 ± 0.15cd | 48.82 ± 2.50cd | 5.12 ± 0.60i | 17.54 ± 2.07g | 1.09 ± 0.08e-g | 18.58 ± 3.49gh | 2.04 ± 0.74bc | 06.92 ± 1.14f |
| Bacillus subtilis 189 | 2.01 ± 0.08cd | 46.17 ± 2.36ef | 6.02 ± 0.70d-f | 42.83 ± 2.30cd | 1.24 ± 0.67cd | 39.43 ± 2.43c | 2.64 ± 0.13ab | 43.71 ± 6.53d |
| Bacillus thuringiensis 199 | 1.67 ± 0.11e-g | 28.19 ± 2.42h | 5.78 ± 0.82fg | 38.46 ± 3.69de | 1.07 ± 0.07e-g | 18.68 ± 1.08gh | 1.94 ± 0.71bc | 01.46 ± 0.13g |
| Bordetella pertussis 263 | 1.58 ± 0.14gh | 20.86 ± 1.92ij | 5.19 ± 0.61gh | 28.83 ± 1.84ef | 1.15 ± 0.26de | 22.82 ± 1.65fg | 2.090 ± 0.28bc | 4.53 ± 0.56g |
| Burkholderia capacia 337 | 1.80 ± 0.12c-e | 37.68 ± 3.82ef | 5.85 ± 0.32d-g | 29.16 ± 3.27f | 1.18 ± 0.07d-f | 25.86 ± 1.92f | 2.79 ± 0.76ab | 48.72 ± 3.58c |
| Burkholderia cepacia CS8 | 2.26 ± 0.09b | 69.26 ± 3.53a | 6.28 ± 0.53cd | 43.94 ± 2.82c | 1.06 ± 0.13fg | 16.52 ± 1.50gh | 2.87 ± 0.11a | 48.20 ± 2.68c |
| Enterobacter sp. CS2 | 1.83 ± 0.08c-e | 39.87 ± 2.24e | 6.53 ± 0.72bc | 55.98 ± 4.67b | 1.17 ± 0.06d-f | 25.80 ± 2.83f | 2.64 ± 0.28a | 43.42 ± 3.05d |
| Microbacterium lacticum 261 | 1.79 ± 0.08d-f | 36.95 ± 2.63ef | 5.04 ± 0.61hi | 18.01 ± 2.27g | 1.07 ± 0.19g | 14.76 ± 1.47h | 2.17 ± 0.31b | 13.14 ± 2.21e |
| Pseudomonas fluorescens 083 | 1.60 ± 1.05e-g | 23.08 ± 1.05i | 6.05 ± 0.95c-e | 45.88 ± 5.36c | 1.28 ± 1.05bc | 40.26 ± 1.05c | 2.01 ± 0.26bc | 06.15 ± 1.26f |
| Pseudomonas fluorescens RB4 | 2.19 ± 0.09b | 65.15 ± 4.28b | 7.05 ± 0.88a | 68.59 ± 9.21a | 1.34 ± 0.09b | 47.59 ± 3.82b | 3.18 ± 0.51a | 62.45 ± 7.39a |
| Sterilized distilled water | 1.49 ± 1.05gh | 11.40 ± 1.05k | 5.34 ± 0.24g-i | 28.34 ± 2.75f | 1.08 ± 1.05fg | 15.49 ± 1.05gh | 1.97 ± 0.17bc | 04.65 ± 0.85fg |
Table 2: Effect of Bacterial spp. on elicitation of defense related biochemicals in Brassica campestris plants; Values are mean ± standard deviation (n=3). Different letters represent significant difference at (P ≤ 0.05) according to ANOVA and DNMRT, IOUC=Increase over untreated control.
Growth Promoting Attributes of Screened Bacteria
Results for growth promoting attributes exhibited that B. megaterium ZMR-6 was positive for ACCD activity while P. fluorescens RB4 was negative for this property. The both screened rhizobacteria showed capability of phosphate solubilization, siderophore production and auxin synthesis.
Field Experiment
Likewise greenhouse experiment, the screened bacterial spp. showed excellent results against Alternaria leaf spot disease under field trials. Co-inoculation of B. megaterium ZMR-6 and P. fluorescens RB4 proved more successful in management of Alternaria leaf spot disease as compared to individual application of bacterial sp. The screened rhizobacteria also improved growth and yield along with protecting B. campestris plants from leaf spot disorder during field experiments. Bacteria inoculated plants demonstrated significant improvement in plant height and total weight in contrast with un-inoculated control and pathogen treated plants. Co-inoculation of both bacterial spp. endorsed plant length up to 34% and 25% under I and II experiment, respectively. Similarly, B. campestris plants primed with bacterial spp. showed significantly higher number of pods. Thus current observations favor the use of PGPR to improve growth and mange disease of plants under field conditions.
Different beneficial bacteria and fungi may protect plants against biotic and a biotic stress [33-35] has reported that phenolic compounds protect plants from different pathogens. The screened bacterial inducers enhanced production of phenolic compounds (Table 3). Akram and Anjum [23] have demonstrated involvement of antioxidant enzymes (PAL, PPO, PO) in disease resistance. Some other researchers have also revealed role of these enzymes in plant disease resistance [36]. These defense related enzymes play a key role in phenylpropenoid pathway of plants which improve plant vitality and vigor. These enzymes also improve disease resistance in plants by degradation of pectolytic enzymes synthesized by disease causing fungi and bacteria [37,38]. Van Loon [39] found that defense related enzymes increase the formation of lignin which acts as a barrier against pathogen infection. Ryals et al. [40] observed involvement of PAL in the phenylpropanoid pathway. PAL is also play a role in formation of lignin and flavonoids which restrict pathogen infection in plants [41]. PO helps in deposition of polysaccharide along with lignifications and suberization and lignification of plants which reduce the chances of disease development [42,43]. PPO helps in formation of antimicrobial compounds which enhance systemic resistance in plants against disease causing agents [44,45].
| Treatments | Experiment-I | Experiment -II | ||
|---|---|---|---|---|
| Disease index Control effect | Disease index | Control effect | ||
| (%) | (%) | (%) | (%) | |
| BM | 35.72 ± 3.16c | 56.14 ± 5.42b | 26.75 ± 2.34bc | 59.14 ± 4.32b |
| PF | 43.51 ± 4.64b | 45.52 ± 4.63c | 30.26 ± 3.14b | 61.16 ± 4.53b |
| BM ± PF | 21.94 ± 3.92d | 64.43 ± 8.61a | 18.32 ± 1.23d | 73.68 ± 6.24a |
| PC | 79.38 ± 8.61a | ND | 74.65 ± 05.43a | ND |
| UC | ND | ND | ND | ND |
Table 3: Potential of selected bacterial spp. on Alternaria leaf spot management in Brassica campestris under field conditions; Values are mean ± standard deviation (n=3). Different letters represent significant difference at (P ≤ 0.05) according to ANOVA and DNMRT. BM=B. megaterium ZMR-6, PF=fluorescens RB4, PC=Pathogen Control, UC=Untreated Control.
During current study screened bacteria induced improved activity of defense related biochemicals and enzymes in brassica plants resulting enhanced systemic resistance [46,47]. Since each bacterial isolate was obtained from different resource, therefore each bacterium showed difference in disease reduction [33,48]. Our results for improved disease resistance in case of combined application of screened bacteria are in accordance with the findings of [49]. Our results regarding higher production of defense related biochemicals and enzymes in combined bacterial application confirm findings of Raupach and Kloepper [50]. Jetiyanon and Kloepper [51] also reported improved production of defense related metabolites and reduced disease level in plants inoculated with PGPR consortium.
Nihorimbere et al. [52] found enhanced growth in plants assisted with disease reducing microbes. Similarly, Bacon and Hinton (2002) [53] revealed improved plant growth under the influence of bacterial inducers. Our study also showed improved plant growth, biomass production and pod formation in plants assisted with bacterial inducers. PGPR synthesize growth promoting hormones which may improve plant growth [54]. Glick et al. [55] reported that ACCD producing PGPR lowers ethylene production resulting improved biomass production in plants under stress. On the other hand some other scientists observed that siderophore producing rhizobacteria improve iron availability to plants which in return improve growth of plants [56-58]. Our results are also in congruent with findings of Adhikari et al. [52]. Bacon and Hinton [47] also found role of bacterial inducer in improvement of growth in co cultivated plants (Table 4).
| Treatments | Experiment 1 | Experiment 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Plant height | Total weight (g) | Number of | Plant Height | Total weight (g) | Number of | |||
| (cm) | Fresh | Dry | pods | (cm) | Fresh | Dry | pods | |
| BM | 31.42 ± 1.94b | 108.34 ± 7.54bc | 12.45 ± 2.32ab | 17.35 ± 1.74bc | 36.07 ± 3.26b | 123.54 ± 7.82b | 19.36 ± 2.43b | 16.45 ± 2.35c |
| PF | 28.53 ± 2.17bc | 97.48 ± 6.46c-e | 9.82 ± 1.68b-d | 19.63 ± 2.04b | 32.76 ± 2.83bc | 107.16 ± 6.43c | 14.89 ± 1.96c | 13.48 ± 2.62cd |
| BM ± PF | 36.86 ± 3.24a | 117.82 ± 8.63ab | 14.25 ± 1.53a | 25.81 ± 3.46a | 39.45 ± 4.05ab | 148.94 ± 8.76a | 24.68 ± 3.14a | 24.13 ± 1.97a |
| PC | 17.18 ± 1.72e | 48.37 ± 4.25g | 5.94 ± 01.06de | 08.16 ± 0.82d | 19.82 ± 2.46e | 63.56 ± 06.42d | 08.69 ± .93e | 05.65 ± 1.05e |
| UC | 25.68 ± 2.36cd | 89.68 ± 06.96ef | 7.92 ± 01.36cd | 18.73 ± 2.81b | 29.86 ± 2.92cd | 119.53 ± 9.11b | 12.82 ± 1.34cd | 18.94 ± 1.58bc |
Table 4: Effect of bacterial spp. on growth attributes of Brassica campestris under field conditions; Values are mean ± standard deviation (n=3). Different letters represent significant difference at (P ≤ 0.05) according to ANOVA and DNMRT. BM=B. megaterium ZMR-6, PF=fluorescens RB4, PC=Pathogen Control, UC=Untreated Control.
The present study exhibited that B. megaterium ZMR-6 and P. fluorescens RB4 are capable to induce systemic resistance and reduce Alternaria leaf spot disease in brassica. This improved disease resistance may be attributed to enhance production of defense related biochemicals and enzymes in plants under the influence of these rhizobacteria. Moreover, the growth promoting characteristics of B. megaterium ZMR-6 and P. fluorescens RB4 improve growth, biomass production and yield of associated brassica plants in an environmentally safe way.