ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
Alejandra Páez-Sánchez1, Ruth Alfaro-Cuevas-Villanueva2, Raúl Cortés-Martínez3, Nuria Segovia4
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Arsenic concentration was evaluated in water from thermal springs and groundwater wells at Cuitzeo basin, Michoacán State, Mexico. There were analyzed also the physicochemical parameters and major components. Arsenic was determined by atomic absorption spectrometry within hydride generation. Physicochemical parameters and major components were analyzed by conventional laboratory methods. Average arsenic concentration values found ranged between 0.001 and 3.8 mg/L, indicating levels above Mexican and international recommendations for drinking water use in at least one of the sites. Results of physicochemical characterization revealed that almost all the water samples had good quality for drinking and domestic purposes, except those with high concentrations of arsenic, which presumably has a geological origin.
Keywords |
| arsenic, thermal springs, drinking water, Cuitzeo |
I. INTRODUCTION |
| In some areas, as the result of geological composition, the arsenic concentrations that exist naturally in groundwater are elevated. In Mexico, arsenic is naturally found in the water-bearing of the Lagunera Region (States of Coahuila and Durango) in concentrations that exceed 15 times the values recommended by the World Health Organization (WHO), affecting a population of 400 000 farmers [1]. In drinking water, it is rare to find more than 10 mg/L of arsenic, although values of up to 100 mg/L have been reported, whereas the guide value recommended by the WHO is 0.01 mg/L [2]. In Mexico, the maximum permissible level (MPL) of arsenic for drinking water is 0.025 mg/L [3]. Approximately 1 of every 100 people that drink, for a long period, water with an arsenic concentration over 0.05 mg/L will possibly die of cancer associated with arsenic, mainly skin cancer. This proportion reaches a 10% if arsenic concentration exceeds 0.5 mg/L [2]. |
| Studies about the activity of the Quaternary period in the volcanic field of Michoacán and Guanajuato States, Mexico, indicate that the lacustrine river basins of this zone provide important information about the human impact on the environment [4]. According to previous studies [5, 6, 7], the abundance of arsenic in this region is significant and it could be considered as a potential health risk for inhabitants of this zone. For these reasons, this investigation was centered on the study of arsenic levels in thermal springs and drinking water wells located on small communities around Cuitzeo Lake. In addition, other polluting agents present in the groundwater of these communities were also evaluated, by monitoring eight sites to determine the zones where concentrations of such contaminants exceed the recommendations of the current Mexican regulations [3]. |
II. MATERIALS AND METHODS |
| Studied area |
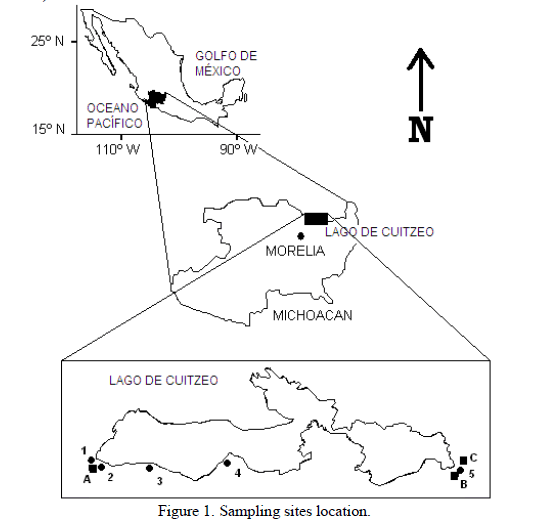
| Cuitzeo Lake is located at the northern part of Michoacán state, Mexico (Fig. 1). The region belongs to the middle part of the Mexican Volcanic Belt [8]. The basin is part of the upper Lerma River and is one of the largest lakes of the zone having an extension of 3,977 km2. The weather is moderate with summer rains (May–October), giving an average annual precipitation of 906 mm and a temperature range from 10âÃâæC to 28âÃâæC. Recent volcanism has occurred in this region; the youngest volcano, Paricutín, was born in 1943. The main regional geological formations are from Tertiary and Quaternary periods. Michoacán hydrology is composed by the upper Lerma river basin, the central lakes zone and the Balsas River. The main landform of the sampling zone is formed by the Cuitzeo depression. The southern region of the lake has been reported to have neutral-alkaline groundwater type. |
| Sampling |
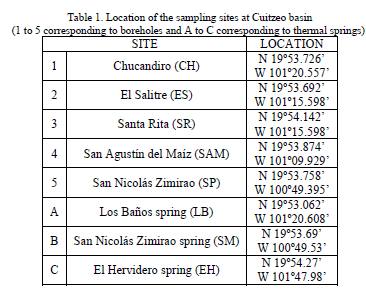
| The sampling sites are located at the south part of Cuitzeo lake, between 100°49´ 53´´ and 101°47´ 98´´ W and 19°53´ 06´´ and 19°54´ 27´´ N, at an average altitude of 1850 m. The samples were obtained from Chucandiro, El Salitre, Santa Rita, San Agustin del Maiz and San Nicolas Zimirao boreholes, and from Los Baños, San Nicolas Zimirao and El Hervidero thermal springs (Figure 1, Table 1). |
 |
| The sampling campaigns were performed from April to September 2007 according to the Mexican regulations [3] and the Standard Methods [9]. Three sampling campaigns to determine total arsenic in water were performed in 120 mL polyethylene bottles, previously washed and decontaminated with HNO3: one day before the sampling the bottles and covers were rinsed three times and filled with deionized water. At the field, two samples were taken at each site, one with the water sample and another with deionized water used as a field blank. The samples were acidified with ultrapure HNO3. Two sampling campaigns for physicochemical parameters and major components were performed on April and September, due to the stability of the chemical composition in both dry and wet seasons of this kind of waters observed in previous studies [5, 6]. The samples for these analyses were taken in 1L plastic polyethylene bottles filled and tightly closed to avoid degassing. |
 |
| Measurement techniques |
| In the field, pH, electrical conductivity, dissolved oxygen and salinity were determined with a Corning potentiometer calibrated before each measurement. Temperature was determined with by immersion with a Branan thermometer. Arsenic was analyzed using an atomic absorption spectrometer (PerkinElmer AAnalyst 200) with hydride generation system. Physicochemical parameters and major components were determined at the laboratory following the Mexican regulations and the Standard Methods [8]. The accuracy of the analyses was checked by the ionic charge balance, obtaining less than 10% difference. |
III. RESULTS AND DISCUSSION |
| Physicochemical parameters |
| The results of physicochemical parameters for each sampling site: San Agustin del Maiz (SAM), Santa Rita (SR), El Salitre (ES), Los Baños (LB), Chucándiro (CH), El Hervidero (EH), San Nicolás Zimirao borehole (SP) and San Nicolás Zimirao spring (SM), are shown in Tables 2 and 3. |
| Water temperature ranges between 24 and 58 ºC, the site where the lowest temperature was registered was ES on August. The highest was registered in EH site on April and August (Table 2). There is no permissible limit in the current regulation for this parameter, nevertheless it is an important characteristic, because some uses are defined by it (i. e. warm and hot water is used for domestic and recreative purposes), and also because this parameter affects directly the chemical and biological reactions of organisms in water. The increase in temperature decreases the portability of water because at elevated temperature carbon dioxide and other volatile gases which impart taste are expelled [10]. Almost all water samples from wells were colorless, clear and odorless indicating the absence of colloidal substances, suspended and decomposed vegetation; only the ES site (Table 3) shows a relatively high value of suspended solids. In the case of water samples from thermal springs, they were colorless and clear, but some sites hoes significant levels of suspended solids but below the MPL. pH ranges between 6.49 and 8.16. SAM on August was the site where lowest pH was registered and LB in April, registered the highest value for this parameter (Table 2). According to NOM-127-SSA1-1994 regulation [3], pH in drinking water should range between 6.5 and 8.5. All the sites in this study were in the permissible range for this parameter. |
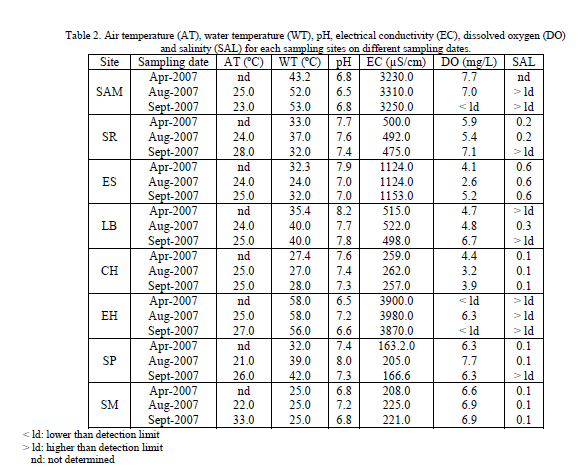 |
| Electrical conductivity (EC) ranged between 163.2 and 3980 μS/cm (Table 2). SP site on April presented the lowest EC value and EH site on august had the highest value for this parameter. There were two sites that stood out for the high EC they presented: SAM and EH. Both sites presented significantly higher values during three samplings than the rest of the sites. Electrical conductivity indicates the presence of dissolved ions and its determination is very important because it gives an idea of the degree of mineralization of water, and it is also related to the Total Dissolved Solids (TDS) in water as it can be clearly seen on Table 3 for the same sites (SAM and EH). The presence of excessive solids in water indicates pollution which can lead to a laxative effect and their presence may be attributed to agricultural activities and geological parameters [10]. |
 |
| nd: not determined |
| Dissolved oxygen (DO) ranged between 2.59 and 7.73mg/L. ES site on August were the site with the lowest DO value and SAM presented the highest one (Table 2). There were two sites that presented values lower than detection limit: SAM on September and EH on April and September. It can be observed a relationship existing between DO and water temperatures, since both sites with values of DO lower than detection limit were the same that presented the highest water temperature. Dissolved oxygen is most important in potable water systems for its effect on other chemicals in the water; it oxidizes both organic and inorganic altering their chemical and physical states and their capacity as a nuisance to the consumer [11]. |
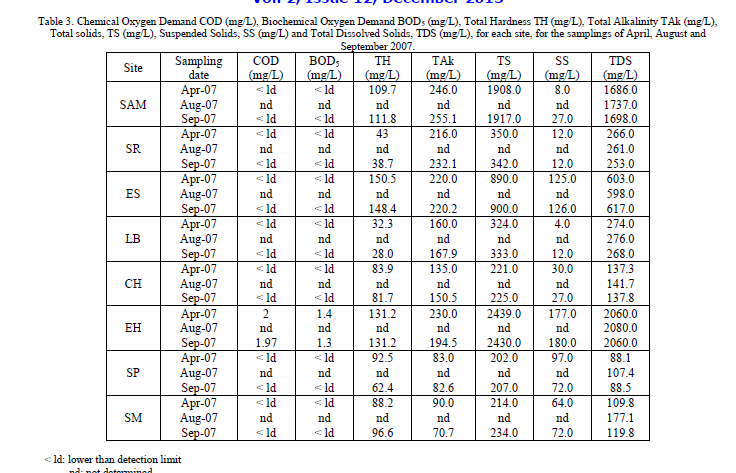
| As for Chemical Oxygen Demand (COD) values, all the sites presented values lower than detection limit, with the exception of the site EH (Table 3), though the values obtained were low (2 mg/L on April and 1.97 on September). COD is the quantity of organic and inorganic matter in water that can be oxidized only by a strong oxidizer and it is used for measuring the degree of pollution caused by organic matter [11]. In this case, the results suggest that pollution due to non biodegradable organic compounds is not significant at these sites. |
| Likewise, the values of BOD5 were found below the detection limit, with the exception of the EH well (Table 3), which values were 1.4 mg/L on April and 1.97 mg/L on September. BOD5 is an estimation of the quantity of oxygen that a microbial heterogeneous population needs to oxidize the organic matter of a water sample in a period of 5 days [12] and as COD it is used to determinate the degree of pollution. These results indicate that only EH site presents organic pollution, though in very low concentrations. |
| Total hardness (TH) ranged between 27.95 and 150.5 mg/L, the highest reported value where found in ES, on April and the lowest one where found in LB on September (Table 3). Total hardness refers to the sum of concentrations of calcium and magnesium. In general, total hardness was lower during rains than during the dry season, with the exception of the sites SAM and SM, where an opposite behavior was observed, and EH, where the values obtained for both samplings were the same. This behavior could be attributed to the dilution effect of rain water, since part of the sampling campaigns where carried out at rainy season. High levels of hardness, may lead to heart diseases and kidney stone formation, making hard waters unsuitable for drinking, washing, cleaning and laundering [10]. On the other side, there is some indication that very soft waters may have an adverse effect on mineral balance [2]. In this case, waters from the study area are not considered as hard waters, but in sites like LB and CH, where TH concentration was less than 100 mg/L, the waters may have a low buffering capacity and may be more corrosive to water pipes. |
| Total alkalinity ranges from 70.647 to 255.125 mg/L. The lowest value was reported for SP during September, while the highest value was reported for SAM also during September. Alkalinity expresses the presence of substances that can be hydrolyzed in water and as a product of the hydrolysis they generate the hydroxyl ion (OH-), like strong bases and the hydroxides of alkaline-earth metals, and also carbonates and phosphates. The presence of borates and silicates in high concentrations also contribute to the alkalinity of the water. High alkalinity values could lead to sour taste and salinity. It can be observed that in sites with a high salinity values, the total alkalinity is also high (Tables 2 and 3). Temporary trend for this parameter shows a slightly increase in concentration during rainy season in sites corresponding to wells and the opposite for thermal springs, which could be attributed to lixiviation of minerals during the recharge of the aquifer. |
| Total dissolved solids ranged between 88.1 and 2080 mg/L, being SP on April, where the lowest value was founded, and EH in August, where the highest value was observed. There were two sites that presented values over the permissible limits (1000 mg/L) and they were SAM and EH, which during three samplings presented similar values and always exceeded the Mexican regulations (DOF, 1994). These results suggest a high presence of inorganic salts, mainly calcium, magnesium, potassium, sodium, bicarbonates, chlorides and sulfates, in waters from these sites. |
| Major ions |
| Major ions constitute a significant part of the total dissolved solids present in groundwater. The results for the concentrations of major ions are shown in Table 4. The content of calcium in the samples ranged between 8.6 and 50.74 mg/L, being LB on September and SP on April where the lowest values were found. In general, the content of calcium of the samples was comparatively low than other ions. Magnesium was found ranging between 1.044 to 16.708 mg/L. The lowest value was reported for three sites: SR, on both samplings; LB on April and EH on September. The highest value was reported for ES on April. The magnesium was also found in relatively low concentrations. Generally, the sites that presented the highest calcium and magnesium concentrations were also the sites with the highest values of total hardness (Table 3). The abundance of Ca, Na, and Mg is associated with minerals such as montmorillonite, illite, and chlorite [13]. Calcium ion present in the ground water of the study area might have come from dissolution of precipitates of CaCO3 and CaMg(CO3)2 during recharge. Magnesium may have come from the dissolution of magnesium calcite, gypsum, and/or dolomite [13, 14]. |
| Sodium ranged from lower than detection limit up to 806.782 mg/L. The site that reported a lower than detection limit value where SP on April and the highest value where found in EH also in April. Sodium was the predominant cation in SAM in both samplings. There are three sites that surpassed the MPL allowed by Mexican regulations (200 mg/L) [3]: SAM, EH and ES. There is a direct relationship between high concentrations of sodium and coronary diseases, hypertension and renal and hepatic diseases [15]. Waters from these sites should not be used as a drinking water source due to their high content of sodium. |
| Chloride occurs in all natural waters in varying concentrations. Concentration is usually greater in groundwater than surface water especially if salt deposits are in the area. Chloride in small concentrations are not harmful to humans in drinking water, and with some adaptation, the human body can tolerate water with as much as 200 mg/L chloride ion [11]. In sites from the study area, Chlorides ranged between 3.39 to 999.69 mg/L. SP site on April, presented the lowest value and EH site on September registered the highest value. Chlorides were the predominant ion in EH and SAM. Also, these two sites exceeded the maximum concentration allowed by the Mexican regulations (250 mg/L) [3]. In water containing 25 mg Cl-/L it can be detected a salty flavor if the predominant cation is sodium, which in this case, a strong correlation can be observed between these ions (Na+ and Cl-), Table 5, specially on sites SAM and EH. On the other hand, this flavor can be absent in water containing even 1 g/L of Cl-, when the predominant cations are calcium and magnesium. Furthermore, high concentrations of chlorides can and avoid the growth of plants and can cause damages to metallic structures by promoting corrosion of pipes, which could be potentially dangerous if pipes are made of toxic metals. |
| Sulphate is one of the least toxic anions of which WHO does not have any recommended value for drinking water, but catharsis, dehydration and gastrointestinal irritation have been linked to high sulphate concentrations in drinking water [11]. In addition, values over 250 mg/L have a "purgative" effect [16]. In this study, sulfates ranged between 2.25 and 450.244 mg/L. The lowest value was found in SP on September and the highest value corresponded to SAM on the same month. Only SAM surpassed the MPL allowed by Mexican regulations [3]. In the water systems for domestic use, sulfates do not produce an increase of corrosion on metallic accessories. Nevertheless, when sulfates concentrations are over 200 mg/L, the concentration of dissolved lead from lead pipelines is increased. |
| Bicarbonates ranged between 83 and 255.125 mg/L. The lowest value was found in SP on April and the highest one in SAM on September (Table 4). Bicarbonates were the predominant ions in all the sites with the exception of SAM and EH. Carbonates were not reported with the exception of two sites (SR and LB, both on September) and in very low concentrations (1.834 mg/L) (Table 4). Bicarbonate were the predominant ion due to the fact that carbonates are only found in water which pH is over 8.3 (carbonates were only found in two of the sites with highest pH values). |
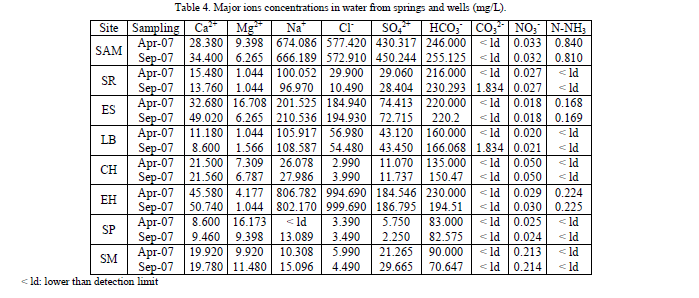 |
| Nitric oxides, nitrates and ammonia/ammonium are very soluble in water and can quickly end up in ground and drinking water. Nitrates in sampled sites were found in concentrations from 0.018 up to 0.214 mg/L. The lowest value corresponded to ES on September and the highest one corresponded to SM also on September. Ammonia nitrogen was only found in SAM, ES and EH, in both samplings. Concentrations ranged from 0.168 to 0.84 mg/L. ES on April presented the lowest value and SAM also on April presented the highest one. This last site presented concentrations that exceeded Mexican regulations [3]. The toxicity of the ammonia nitrogen depends on the ionic composition, temperature and pH. Particularly to acid pH, the toxicity of ammonia nitrogen increases hundred times, because it takes the not ionized form, or, ammonia. On the other hand, when pH is higher than 10, the whole ammonia nitrogen is ionized and presented as ammonium [17]. |
| Arsenic |
| The results for the content of arsenic for each sampling site, during the three samplings are shown in Table 5. The content of arsenic in LB and EH, was over the maximum permissible level of the in force Mexican regulations for water destined for human use and consumption [3]. Concentrations of arsenic ranged between 0.001 and 3.812 mg/L and some samples revealed a content of arsenic lower than the detection limit of the instrument (CH on September, 2007 and SM in the three samplings). |
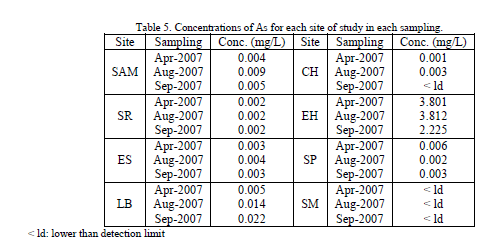 |
| The site that presented the lowest value was CH on April (0.001 mg/L) and the one that presented the highest value was EH on August (3.812 mg/L). Nevertheless in EH, during the whole period of study, the concentrations exceeded the MPL for water destined for human use and consumption [3] by several orders of magnitude. Another site that exceeded, the guidelines for arsenic in drinking water [2, 3] was LB during August and September (0.014 and 0.022 mg/L respectively). SAM during August almost reached the MPL (0.009 mg/L). |
| The upgrade to the NOM-127-SSA1-1994 Mexican norm, regarding to environmental Health, water for human use and consumption, permissible limits of quality and treatments for water purification [18], indicates as maximum permissible limit a concentration of 0.01 mg/L of arsenic since 2006, whereas the water collected in the spring El Hervidero (EH) the lowest concentration was of 2.225 mg/L (September, 2007) and the highest was of 3.812 mg/L (August, 2007). Natural enrichment of groundwater by arsenic can arise in several ways like hydrothermal volcanism, oxidation of arsenical sulphide minerals, reduction of FeOOH and release of its sorbed load to groundwater, desorption of As from mineral sorption sites in response to increase of pH, and evaporative concentration; as well as anthropogenic sources like agricultural applications, wood preservation, glass production and mining activities [19, 20, 21]. On the EH site, there is not industrial or significant agricultural activities, therefore these sources can be discarded as pollution sources for this site. Besides, some studies have found presence of arsenic in groundwater near the studied area (used mainly for geothermal exploitation), relating As occurrence to the water-rock interaction and the geothermal nature of the zone [22, 23]. Furthermore, a general relationship between As and salinity in geothermal waters from the USA has been reported [21, 24], where concentration greater than 1 mg/L of As mostly had Cl- concentrations of 800 mg/L or more, which is the case of EH site in this study. |
| Water uses |
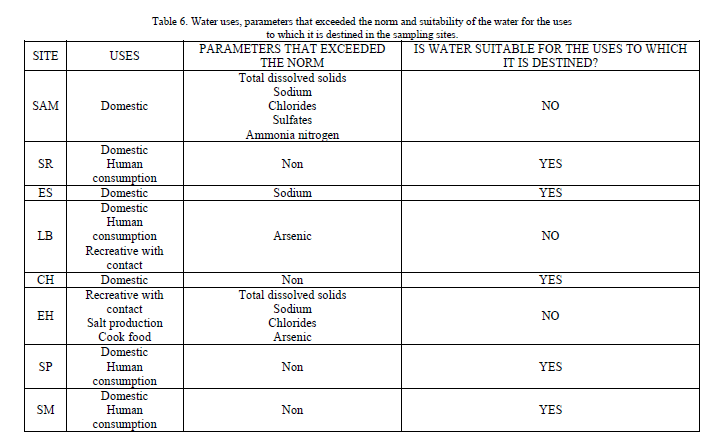
| As for the uses to which the water of every analyzed source is destined (domestic, human consumption, recreative with contact and salt production), these are shown in Table 6. |
 |
IV. CONCLUSIONS |
| Generally, physicochemical quality of water from springs and wells at the studied area is good, with some exceptions like SAM site that has a major quantity of parameters that exceed the Mexican regulations, but, due to the fact that the use that is given to this water is only domestic, it does not represent any risk for the health, though the high concentration of chlorides could damage the pipelines and other metallic structures. Besides, in LB site, the parameter that exceeded the permissible limit was arsenic and due to the fact that the water in this site is used for domestic activities, for human consumption and recreational activities with contact, it represents a risk for the health of the people who uses and consumes this water. Therefore, a treatment process for the removal of arsenic is recommended. |
| In EH site, total dissolved solids exceeded the permissible limit, as well as sodium and chlorine, but this doesn’t represent any risk for the health, since the use of the water is recreative with contact and for obvious reasons, for the extraction of salt. Nevertheless, this site presented the highest concentrations of arsenic found in this study, and they exceeded for much the permissible levels of Mexican and international regulations. Hence, it is not recommended its use for any practical uses before a water treatment is applied. |
| The sites SR, CH, SP and SM did not show parameters that were exceeding the regulations, for what it is considered that this water is totally suitable for any of the uses for which it is destined. |
ACKNOWLEDGMENT |
| The authors would like to acknowledge financial support by Coordinación de la Investigación Científica (CIC-UMSNH, 2013), in the same way Juan Rangel Camarena and Benjamín Villalobos Castañeda for technical assistance. |
References |
|