Keywords
|
| Retinal fundus image, Optic Disc, Microaneurysms, Haemorrhages, Exudates, mathematical morphology, Top-hat Transform |
INTRODUCTION
|
| Diabetes mellitus [1], commonly referred to as diabetes describes a group of metabolic diseases in which the person has high blood glucose (blood sugar), either because insulin production is inadequate (Type 1 Diabetes), or because the body's cells do not respond properly to insulin (Type 2 Diabetes), or both. Diabetes leads to a variety of pathologies in the body namely Diabetic Neuropathy [2], Diabetic Nephropathy [3], and Diabetic Retinopathy [4-9]. Diabetic retinopathy results due to the damage to the tiny blood vessels that supply nutrients to the retina [6]. They leak blood and other fluids such as lipid proteins, fats etc. that cause swelling of retinal tissue and affects normal vision. It usually affects both eyes. The longer the patient has Diabetes there is more likelihood that the person will develop the Diabetic Retinopathy. If Diabetic Retinopathy is not treated for long time it can cause permanent blindness [5].Presently diabetic retinopathy can be accounted for approximately 5% of global blindness [4]. |
| Based on the damage done to the retina the disease is classified into two stages. Non-proliferative diabetic retinopathy (NPDR) is the early state of the disease in which there are no or minimal symptoms of disease. In NPDR, the blood vessels in the retina are weakened causing tiny bulges called micro-aneurysms [5] to protrude from their walls. These tiny bulges may leak blood or other fluids such as fats or proteins.Proliferative diabetic retinopathy (PDR) [5] is the more advanced form of the disease. At this stage, the retina becomes severely oxygen deprived and so new blood vessels starts forming to supply oxygen to the retina. These new vessels are abnormal so they are very fragile and tends to burst, leaking blood into the vitreous (fluid that fills the space in front of retina) clouding vision through a process called vitreous haemorrhage [5]. |
| Often there are no visual symptoms in the early stages of diabetic retinopathy. Early detection and treatment can limit the potential for significant vision loss from diabetic retinopathy. Both people with Type 1 as well as Type 2 diabetes are at risk for the development of diabetic retinopathy. The longer a person has diabetes, the more likely they are to develop diabetic retinopathy, particularly if the diabetes is poorly controlled. In the early stages of Non-proliferative Diabetic Retinopathy, treatment other than regular monitoring may not be required. Following the doctor's advice for diet and exercise and keeping blood sugar levels well-controlled can help control the progression of the disease. That is why the early detection of Diabetic Retinopathy and regular eye checking is extremely important for all the diabetes patients [9]. |
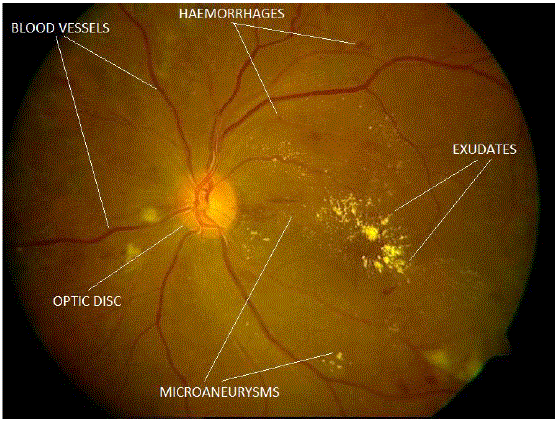
| Micro aneurysms, Haemorrhages and Exudates are the Characteristic features seen at different stages of Non Proliferative Diabetic Retinopathy [5]. Microaneurysms are the first sign of Diabetic Retinopathy which can be observed on the retinal fundus image. As the disease progresses some of the capillaries rupture and appear as small dots or larger blots or flame-shaped haemorrhages [8] which appear red on fundus image. If these capillaries leak precipitates of lipids, serum proteins then it will lead to yellow lesions called as exudates [5]. Based on the extent of the presence of these features the diabetic retinopathy is classified into Mild Non-proliferative Diabetic Retinopathy, Moderate Non-proliferative Diabetic Retinopathy, and Severe Non-proliferative Diabetic Retinopathy. |
| Various methods have been used for segmentation of the diabetic features. Segmentation of exudates using mathematical morphology [11] has been proved particularly useful [12-19]. Morphological Top-Hat transform [17], edge detection [18, 19] and recursive region growing [14, 15] are some widely used methods for segmentation of microaneurysms and haemorrhages. |
RELATED WORK
|
| Ramanuka et al [12] used morphological operations to detect hard exudates. Fuzzy logic was further used to classify the hard exudates. Zhang et al [13] used separate methods for small and large exudates. Large exudate candidates were obtained by applying mean filter on the preprocessed image followed by a reconstruction. Morphological top-hat transform was used to detect the small exudates. |
| Sopharak et al [14] used local variance operator and morphological operations such as opening, closing and reconstruction to extract exudates. Otsu algorithm was used to determine the thresholds. Sinthanayothin [15, 16], used the recursive region growing approach to separate exudates which essentially has the sharp edges. Further for extracting the microaneurysms and Haemorrhages a Moat operator was used to create sharp edges in the image on which recursive region growing technique was used. Spencer et al [17] used bilinear top-hat transformation to detect microaneurysms from a shade corrected image. |
PROPOSED ALGORITHM
|
| The green channel of the image is selected for the segmentation of the features both normal and abnormal because in has the maximum contrast than the original or red or blue channel. Then the contrast of the green channel is increased by using either contrast limited adaptive histogram equalization [CLAHE] or histogram equalization [11] depending upon suitability. |
| A. Optic Disc Detection |
| The optic disc corresponds to the brightest component of the retinal image. The intensity levels of optic disc are very similar to that of exudates so it is very important to detect the optic disc separately. Let I1 be the grayscale image of the green channel of the original image. Image I1 is pre-processed using histogram equalization to get image I2. Candidate optic disc region is then separated from I2 by segmenting the region of maximum intensity. Image I3 is then obtained by applying a 3x3 median filter to remove the stray pixels (Salt and Pepper noise). Then a morphological opening operation (o) using a flat disc shaped structuring element (D1) is performed on I3. |
| I4 = I3 0 D1 |
| A Suitable Structuring element is chosen that differentiates between the optic disc and exudates based on size. It is assumed here that any stray exudates detected are smaller than the size of optic disc. The opening operation will eliminate the smaller exudates present if any. |
| B. Blood Vessel Detection: |
| Blood vessel segmentation is a significant step in the red lesion detection. Since the blood vessels and red lesions namely microaneurysms and haemorrhages are both red in colour the blood vessels need to be extracted out of the fundus image in order to effectively detect the microaneurysms and haemorrhages. A Contrast limited adaptive histogram equalization (CLAHE) is performed on the negative of I2 to get resultant image I5. Image I6 is obtained by performing Top-hat filter operation on image I5 using a flat disc shaped structuring element (D2). Top-hat filtering is the equivalent of subtracting the result of performing a morphological opening operation on the input image from the input image itself. |
| I6 = I5 - (I5 o D2) |
| Suitable threshold is used to segment out the blood vessels from image I6 This threshold is selected based on the apriori knowledge of the quality of the image. The resultant image (I7) comprises of blood vessels along with haemorrhages, micro-aneurisms and other stray structures. After removing structures that have area less than a decided threshold we get image I8 containing only blood vessels. |
| |
| C. Exudate detection: |
| Exudate detection is a complicated task due to the presence of many bright structures which can be mistaken into exudates. They are optic disc, Optic nerve fibres, reflections in the middle of the vessels and reflections which are present particularly on retina of young patients [13]. However, most of these structures especially optic nerve fibres do not have edges as sharp as exudates. False detection of non-exudate feature is avoided by using a unique technique involving edge detection, morphological closing and logical AND operation. Sharp edges were extracted from image I1 using Sobel filter to obtain image I8 .A morphological closing operation (⋅) is performed on I8 to fill the edges using a flat disc shaped structuring element (D3). |
| I9 = I8 o D3 |
| The CLAHE is applied on the image I1 subsequently bright region is extracted using a suitable threshold to get image I10. Logical AND operation is then performed between image I10 and image I9. |
| I11 = I10 and I9 |
| The Optic disc then subtracted from the resultant image to give the exudates. |
| I12 = I11 - I4 |
| D. Microaneurisms and Haemorrhage Detection: |
| The microaneurysms and dot haemorrhages are Structures with sharp edges and circular in shape [18]. I13 is obtained by applying Prewitt edge detector on I1. The detected edges are then filled by applying morphological closing operation by using a flat disc shaped structuring element (D4). |
| I14 = I13 . D4 |
| The image obtained contains microaneurysms and Haemorrhages and blood vessels.Logical AND operation is then performed between image D14 and image I7. |
| I15 = I14 and I7 |
| This step removes the false detection of the red lesions. Finally the blood vessels (I8) are subtracted from (I15) to get the red lesions. |
| I16 = I15 - I8 |
| Microaneurysms and haemorrhages are further separated from each other depending on the difference in their sizes. Structures having area smaller than a threshold are classified as candidate microaneurysms (I17). The candidate microaneurysms contain small fragments of blood vessels along with microaneurysms. Since these fragments are thin linear structures these are eliminated by applying a morphological opening operation with a disc shaped structuring element (D5). Structures bigger than microaneurysms are classified as candidate haemorrhages. These also contains fragments of blood vessels which are eliminated by applying a morphological opening operation with a disc shaped structuring element (D6) having radius greater than that of D5. |
RESULTS
|
| Two different images are considered. One image is used for detection of the optic disc and exudatesanother one is used for the detection of blood vessels, microaneurysms and haemorrhages. |
| a. Optic Disc Detection |
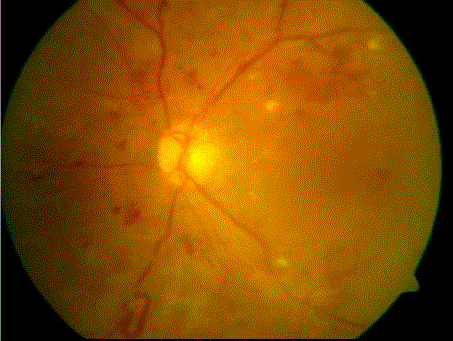
| Input fundus image shown is shown in figure 2a.Gray scale image of the green channel of input image is shown in figure 2b.After applying histogram equalization on the image figure 2b we get contrast enhanced image figure 2c. Segmenting out the region of maximum intensity level gives all the bright regions in the image which contains optic disc and some false detection which typically from small exudates. These features are removed by performing several morphological opening operations. The obtained image is then morphologically dilated by using a flat disc shaped structuring element of small radius. Figure 2d shows the detected optic disc. |
| b. Blood Vessel Detection |
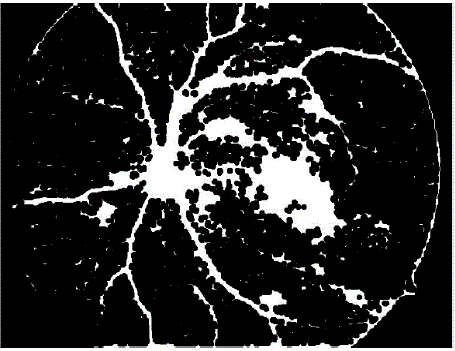
| The image shown in figure 3a isconsidered as the input image for blood vessel detection.The inverted histogram equalized image of grayscale image of green channel of input image as shown in figure 3b is filtered using top-hat transform. The top-hat transformed image (figure 3c) is then made binary using a suitable threshold. After binarization blood vessels as well as noise pixels, microaneurysms and haemorrhages are also present in the image. Suitable area threshold is used to remove these small structures. After removal of small objects we get the blood vessels as shown in figure 3d. |
| c. Exudates Detection |
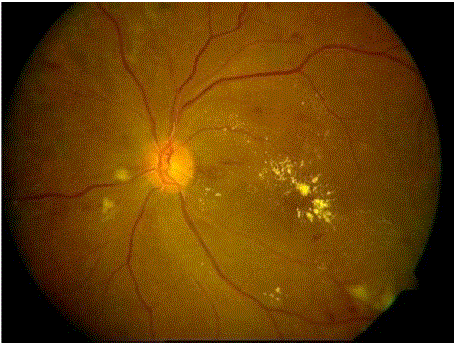
| Image shown in figure 2a is considered as the input image and a Sobel edge detector is used to find the edges. Figure 4a shows the closed edges, these typically involve blood vessels, microaneurysms, haemorrhages and exudates. Logical AND operation is performed between the image with bright region shown in figure 4b and figure 4a. This gives output as in figure 4c which contains some bright pixels in the optic disc region. From this image the earlier segmented Optic disc is subtracted to get the exudates as shown in figure 4d. |
| d. Microaneurysm and haemorrhage Detection |
| Image shown in figure 3ais considered as the input image. The edges detected by Prewitt filter after filling with closing operation contains blood vessels, exudates, microaneurysms and haemorrhages and is shown in figure 5a. Logical AND operation is then performed between this image and image 3dto get Figure 5b. Blood vessels are then subtracted from resultant image to give microaneurysms and haemorrhages with presence of noise pixels and small fragments of blood vessels. Small noise pixels are eliminated by performing a morphological opening operation by a disc shaped structuring element and small radius. Small structures within normal range of MAs are considered as MAs and separated from rest and shown in figure 5c. Larger fragments of blood vessels are linear structures, so when morphological opening operation with little larger radius is performed these fragments are removed leaving only true dot haemorrhages as shown in figure 5d. |
CONCLUSION AND FUTURE WORK
|
| The fast and efficient early detection of Diabetic Retinopathy is only possible if there is an effective method for segmenting the diabetic features in the fundus image. The above proposed methods presents a fast, effective and robust way of detecting diabetic features in the fundus images which can be used for classification of the images based on the severity of the disease. Using the extracted features a classifier can be designed which will classify the images into several categories. Since manually detecting the presence of diabetic features especially tiny microaneurysms and haemorrhages is very difficult the proposed method can be of great help to the ophthalmologists’ for mass screening of patients. |
| |
Figures at a glance
|
 |
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
Figure 5 |
|
| |
References
|
- World Health Organization.Department of Noncommunicable Disease Surveillance, “Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications” Report of a WHO Consultation, WHO/NCD/NCS/99.2, Vol.No. 1,1-59, 1999.
- M. Pasnoor, M. Dimachkie, P. Kluding and R. Barohn, “Diabetic neuropathy part 1: Overview and symmetric phenotypes”,NeurolClin.,Vol.No.31, Issue No.2, 425–445,2013.
- P. K. Dabla, “Renal function in diabetic nephropathy”,World J Diabetes, Vol. No.1, Issue No.2, 48-56,2010.
- RaczyNska, K. Zorena, B.Urban, D. Zalewski, A. Skorek, G. Malukiewicz and B. L. Sikorski, “Current Trends in the Monitoring and Treatment of Diabetic Retinopathy in Young Adults”, Mediators of Inflammation, 1-13,2014.
- A. A. Alghadyan, “Diabetic retinopathy – An update” Saudi Journal of Ophthalmology, Vol.No.25, 99–111, 2011.
- M.D. Abràmoff, M.K. Garvin and M. Sonka, “Retinal Imaging and Image Analysis”,IEEE Trans Med Imaging,Vol.No. 3, 169–208,2010.
- T. Gardner, S. Abcouwer, A. Barber and G. Jackson, “An Integrated Approach to Diabetic Retinopathy Research” ,Arch Ophthalmol, Vol. No.129, Issue No.2, 230–235,2011.
- K Viswanathand D. McGavin, “Diabetic Retinopathy: Clinical Findings and Management”,Community Eye Health, Vol.No.16, Issue No.46, 21–24,2003.
- L. Wu, P. Fernandez-Loaiza, J. Sauma, E. Hernandez-Bogantes and M. Masis, “Classification of diabetic retinopathy and diabetic macular edema”,World Journal of Diabetes, Vol. No.4, Issue No.6, 290-294,2013.
- T. Walter, J. Klein, P. Massin and A. Erginay, “A Contribution of Image Processing to the Diagnosis of Diabetic Retinopathy—Detection of Exudates in Color Fundus Images of the Human Retina”, IEEE Transactions on medical imaging, Vol. No. 21, Issue No. 10, 2002.
- Gonzalez, Woods and Eddins,Digital Image Processing Using MATLAB. 1st ed. India: Pearson Education Inc. and Dorling Kindersley (India) Pvt. Ltd. 2006.
- R.H.N.G.Ramanukaand R.G.N. Meegama, “Detection of hard exudates from diabetic retinopathy images”, PNCTM, Vol. No.1, 34-39, 2012.
- X. Zhang,G. Thibault, E. Decencière, B. Marcotegui, B. Lay, R. Danno, G. Cazuguel, G. Quellec, M. Lamard, P. Massin, A.Chabouis, Z. Victor, andA.Erginay, “Exudate detection in color retinal images for mass screening of diabetic retinopathy”,Medical Image Analysis, 1026– 1043, 2014.
- A. Sopharak, B. Uyyanonvara, S. Barmanb and H. Williamson, “Automatic detection of diabetic retinopathy exudates from non-dilated retinal images using mathematical morphology methods “,Computerized Medical Imaging and Graphics Vol. No.32, 720–727, 2008.
- A. Sinthanayothin, J.Boyce, T. Williamson, H. Cook, E. Mensah, S. Lal and D. Usher, “Automated detection of diabetic retinopathy on digital fundus images”, Diabetes UK Diabetic Medicine, Vol.No.19, 105–112,2002.
- A. Sinthanayothin, J. Boyce, H.Cookand T. Williamson, “Automated localisation of the optic disc, fovea, and retinal blood vessels from digital colour fundus images”,Br J Ophthalmol, Vol. No.83, 902–910, 1999.
- T. Spencer, J. Olson, K.Mchardy, P. Sharp and J. Forrester, “An Image-Processing Strategy for the Segmentation and Quantification of Microaneurysms in Fluorescein Angiograms of the Ocular Fundus” Computers and biomedical research, Vol. No. 29, 284–302, 1996.
- S. Ravishankar, A. Jain and A. Mittal, “Automated feature extraction for early detection of diabetic retinopathy in fundus images Proceedings” IEEE Computer Society Conference on Computer Vision and Pattern Recognition , 210-217, 2009.
- S. Prabhu, C. Chakraborty, R.N. Banerjee and A. K. Ray, “Study of Retinal Biometric Systems with respect to Feature Classification for recognition and Diabetic Retinopathy”, Journal of Medical Imaging and Health Informatics,Vol. No.1, 1–10, 2011.
|