ISSN: 2319-9873
ISSN: 2319-9873
1Fuels and Combustion Research Laboratory, School of Nano Science and Technology, National Institute of Technology Calicut 673 601, India
2Department of Mechanical Engineering, National Institute of Technology, Calicut 673 601, India
Received: 08 May 2015 Accepted: 15 May 2015 Published: 22 May 2015
Visit for more related articles at Research & Reviews: Journal of Engineering and Technology
Harmful emissions associated with the use of biodiesel is a serious issue and various fuel additives are being used for the reduction of emissions as well as for the improvement of engine performance. Use of cerium oxide nanoparticles as fuel additive is one of the methods for the reduction of emissions, due to its peculiar redox functionality and oxygen buffering capability. Doping of ceria with transition metals such as zirconium improves its Oxygen storage capacity and thermal stability, thereby enhancing simultaneous oxidation and reduction reactions. The present work focuses on the development of cerium zirconium mixed oxide nanoparticle based additive for the reduction of emissions from diesel engine fuelled with biodiesel - diesel blends. Cerium zirconium mixed oxide was synthesized by means of co precipitation method. The stability of the nanofluids was improved by the addition of surfactant, namely Oleic acid. The optimum concentration of surfactant was determined based on estimation of critical micelle concentration, by means of standard tests. Stability of catalytic nanoparticle in fuel was evaluated from the measurement of Zeta potential. Various properties were determined as per ASTM standards to investigate the effect of the nanoparticles on fuel properties. Addition of catalytic nanoparticle in diesel - biodiesel blends does not significantly affect the fuel properties. Engine performance and emission tests were conducted on single cylinder diesel engine to assess the potential of synthesized nanofuel and 15% average reduction of NO emissions was observed for B5 and B10 blends with 15 ppm of catalytic nanoparticle concentration.
Cerium, Zirconium, Nanoparticle, Biodiesel, Emission.
The diesel engines are employed in the power sector for diverse applications due to better fuel economy and power output. However inherent tailpipe emissions of NOx and particulate matter (PM) associated with them poses a great threat to human health and environment. Improvement in the diesel engine performance is indispensible due to severe energy crisis and emission norms, which are getting more stringent. Majority of studies shows that blending of biodiesel results in slight increase in NOx emissions as compared to diesel [1]. HC and NOx emissions in the presence of sunlight react to form photochemical smog and acid rain which causes respiratory problems and eye irritation in human beings. The development of improved technologies for the reduction of emissions from the diesel engine is of much significance in the present scenario. Emission reduction technologies being employed in the diesel engine can be classified into three categories -application of alternative fuels, modification of combustion processes and exhaust after treatment system.
Blending biodiesel with diesel and the use fuel additives to enhance its combustion characteristics is a promising solution, in this regard. Biodiesel comprises of monoalkyl esters of long chain fatty acids which can be derived from various non-edible oils like Madhuca indica, Jatropha curcas, Pongamia pinnata etc. Synthesis of biodiesel from Crude Jatropha Curcas Oil (CJCO) is usually done by two step method, because of its high fatty acid content. The first step involves acid pre treatment process to reduce free fatty acid content followed by alkaline esterification process. Extensive works have been done on the optimization of biodiesel yield with crude Jatropha curcas oil as feedstock [2-4]. The biodiesel powered diesel engine shows better efficiency due to the oxygen content in the biodiesel, resulting in improvement of combustion process [5]. The reduction in PM emissions with the use of biodiesel is mainly due to lower aromatic and sulphur compounds and higher cetane number of biodiesel [1], in addition to high oxygen content, which results in better oxidation of soot. Majority of literature reported an increase in NOx emissions with biodiesel as compared to diesel. Lebeckas and Slavinskas.[6] reported that there is proportional increase in NOx emission with percent of oxygen in rapeseed methyl ester. Sahoo et al. [7] tested biodiesel derived from three different feed stocks in a watercooled three cylinder tractor engine. Maximum enhancement in power was obtained for B50 blend of jatropha biodiesel. Jatropha biodiesel blends showed an increasing trend for CO emissions, whereas karanja biodiesel showed a decrease in CO emissions. However studies conducted by Aydin and Bayindir [8] showed a decreasing trend for CO emissions with cottonseed oil methyl, due to cleaner and complete combustion. Significant decrease in HC emissions was reported with biodiesel from different feed stocks [7].
Various experimental investigations were reported on fuel additives for enhancing diesel engine performance and reduction of the exhaust emissions. In the study conducted by Uner et al. [9], soot oxidation ability of cobalt and lead based mixed oxide catalysts were investigated and it was observed that these mixed oxides decrease the peak combustion temperature of soot by 190ºC and 115ºC, with and without platinum impregnation respectively. The study also revealed that soot oxidation occurs mainly due to the lattice oxygen in the catalyst. Rao et al.[10] used oxygenated compound, Triacetin as an antiknock additive in coconut oil based biodiesel and 10% blend of Triacetin was found to be optimum concentration for the maximum reduction of HC and NO emissions. Keskin et al.[11] investigated the effect of Mg and Mo based additives on B60 blend of biodiesel on properties, performance and emission characteristics. Addition of 12μmol/l Mg additives showed a decrease of pour point and kinematic viscosity by 57% and 17%, respectively, whereas Mo additives showed a decrease of 71% and 19% on pour point and kinematic viscosity respectively. It was also concluded that the biodiesel blends with Mg and Mo has better effect on reduction of PM emissions due to their catalytic effect. Varatharajan et al.[12] studied the effect of anti-oxidants in jatropha biodiesel on emissions of a diesel engine and observed that antioxidants in biodiesel are effective in controling NOx but it gives slightly higher CO and HC emissions.
Lenin et al.[13] investigated the feasibility of metal oxides of Manganese and Copper in nano sized form as diesel additives. Manganese oxide additive resulted in the reduction of CO and NOx by 37 % and 4 % respectively. Among various metal oxides Ceria, especially in nano sized form, is an excellent catalyst for the reduction of emissions and enhancement of efficiency. The catalytic activity of ceria based materials are mainly due to two factors namely Oxygen storage/ redox capacity and surface area enhancement [14]. Cerium oxide in nano sized form has high surface to volume ratio, leading to excellent oxygen storage capacity, thereby causing simultaneous oxidation and reduction of emissions. Cerium oxide undergo transformation from stoichiometric CeO2 (+4) state to the Ce2O3 (+3) valence state via a relatively lower energy reactions.[15]. Doping of ceria with zirconium enables the inhibition of the sintering of ceria and hence provides thermal stability to the catalyst [16], in addition to the improvement of Oxygen storage capacity (OSC). Increase in OSC is mainly due to the occurrence of the bulk defect. The addition of 80 ppm Cerium oxide nanoparticles in biodiesel results in nearly 5% increase in flash point whereas there is no significant effect on cold temperature properties of biodiesel upon the addition of nanoparticle[17] . Arul Mozhi Selvan et al.[18] experimentally investigated the application of Cerium oxide in diesel and diesel-biodiesel-ethanol blends and about 9% decrease in specific fuel consumption was observed. Even though decrease in smoke, CO, and HC emissions was observed with the addition of ceria, NO emission was found to be increased.
Nanofluids are stable fluid suspensions of nanometer-sized particles, instead of the simple particle-liquid mixtures. There are two primary methods for the synthesis of nanofluids, namely single step and the two step method. The single step method consists of synthesizing and dispersing the nanoparticles in the fluid, simultaneously. Chang et al. [19] prepared TiO2-water nanofluids by single step chemical method using a high pressure homogenizer. Lo et al. [20] determined the optimal parameters for the preparation of copper oxide nanofluid by means of submerged arc nanoparticle synthesis system (SANSS) using a copper electrode. In two step method, nanoparticles are first produced as dry powders by chemical or physical methods and then dispersed into a fluid in the second step by means of intensive magnetic force agitation, ultrasonic agitation and so on. Lee et al. [21] produced oxide nanofluids by two step method, in which first A12O3 and CuO nanoparticles were prepared by gas condensation and were dispersed in water and ethylene glycol followed by thorough shaking to ensure a homogeneous suspension. Abareshi et al. [22] prepared nanofluids by dispersing Fe3O4 nanoparticles synthesized by co precipitation method, in deionized water with Tetramethyl ammonium hydroxide as dispersant. W. Yu et al. [23] synthesized stable kerosene based Fe3O4 nanofluids by phase transfer method in which Oleic acid was successfully grafted onto the surface of Fe3O4 nanoparticles by chemisorbed mode, which ensures good stability of nanofluids.
The stability of nanoparticle dispersed in the base fluid is one of the major issues as far as the practical application of nanofluids is concerned. Laura et al. [24] employed different dispersion techniques like sonication, ball milling and high-pressure homogenization to prepare stable water based nanofluids and high-pressure homogenization method was found to be optimum one. Various techniques are being used for the estimation of the stability of nanofluids. Sedimentation method is the most elementary method for the determination of stability of nanofluids, in which sediment weight or the sediment volume of nanoparticles in nanofluid under an external force field is measured [25]. Singh and Raykar [26] used the centrifugation method to evaluate the stability of silver nanofluids synthesized by the microwave synthesis by reduction of AgNO3, with polyvinylpyrrolidone (PVP) as stabilizing agent. Kim et al.[27] used zeta potential to study the stability of gold nanoparticle suspension in water and concluded that characteristic stability is due to the fact that gold nanoparticles are negatively charged. Spectral absorbency analysis is another efficient way to evaluate the stability of nanofluids which gives quantitative results corresponding to concentration of nanofluids. Different techniques are employed for enhancing the stability of nanofluids such as addition of surfactants, surface modification techniques, ultrasonic agitation etc. Surfactants like hexadecyl trimethyl ammonium bromide (CATB) and sodium dodecylbenzenesulfonate (SDBS) significantly increase the stability of Cu - H2O nanofluids by electrostatic repulsions [25].
The present work mainly focuses on the development of a stable catalytic nanoparticle based fuel additive for the reduction of harmful emissions from diesel engine with biodiesel blends and also for the performance enhancement. The catalytic nanoparticle used in the present work is cerium zirconium mixed oxide, which is an excellent catalyst for the reduction of exhaust emissions. Biodiesel was synthesized from Jatropha Curcus oil by two step transesterification process and cerium zirconium mixed oxide nanoparticles were synthesized by co precipitation method. Stabilization of catalytic nanoparticle in diesel - bio diesel blends is one of the major issues in the practical application of cerium zirconium mixed oxide nanoparticle. Use of surfactant is a well established method for improving the stability and has been employed in the present work. The optimum concentration of surfactant for maximum stability was determined based on estimation of critical micelle concentration, by means of standard tests. Stability of catalytic nanoparticles in biodiesel blends was evaluated from the measurement of Zeta potential at various temperatures, by means of Dynamic light scattering system. Various properties of biodiesel - diesel blends, such as density, kinematic viscosity, flash point, cloud and pour point were determined as per ASTM standards to investigate the effect of the nanoparticles on fuel properties. Engine performance and emission tests were conducted on single cylinder diesel engine to assess the potential of synthesized nanofuel.
Biodiesel synthesis
Two step methods were employed for the synthesis of biodiesel from jatropha oil. The first step involves acid esterification in which FFA content is reduced by significant level. The jatropha oil poured was heated in a conical flask and the solution of H2SO4 acid (1% w/w of oil) in methanol (9:1 methanol to oil molar ratio), heated at 50ºC, was added to it. This solution was stirred for 1 hour, keeping the reaction temperature at 50ºC. After 1 hour of reaction, the mixture was allowed to settle for 2 hours and the methanol-water fraction at the top layer was removed. In the second step of synthesis, biodiesel was produced via alkali catalyst based trans esterification using the product obtained in first step. The catalyst NaOH(0.55% w/w of oil) and methanol(6:1 methanol to oil molar ratio) mixture was poured into the acid pre-treated oil, heated up to 60ºC, in the conical flask and stirred for 1.5 hour at 60ºC which yield the reaction products namely, free glycerol and fatty acid esters, known as biodiesel. The reaction product was then allowed to settle for overnight and biodiesel was separated from bottom glycerol layer. As crude biodiesel is normally contaminated mix water, washing method was employed for purification of biodiesel in the present work. Biodiesel was poured into equal quantity of distilled water and this mixture was agitated until it is well mixed and this process was repeated 4 to 5 times. As the mix settles out, the water would slowly sink, taking with it the soluble impurities and the top layer of biodiesel was then separated. The dehydration was also done to get rid of water present in the purified biodiesel. In the first stage, silica gel was used to adsorb water and then it was heated up to 100ºC to remove the remaining traces of water.
Synthesis of Cerium Zirconium mixed oxide
Catalytic nanoparticles used in the present work is cerium zirconium mixed oxide and was synthesized by co precipitation method. 0.1M aqueous solution of precursors, Ammonium Ceric Nitrate & Zirconium Oxychloride was taken in desired ratio and mixed. The prepared solution was stirred for 15 minutes at 60ºC, by using a magnetic stirrer with hot plate. Aqueous ammonia was added drop wise to vigorously stirring solution, till it reaches a pH more than 10. On the addition of aqueous ammonia, solution turned yellow and mixture was stirred for about 2 hours. The precipitates were collected and washed repeatedly with water and acetone to remove excess ammonia .The sample was then dried for 8 hours at 60°C in a vacuum oven. A porous yellow powder was obtained which was grounded in a mortar to get fine powder and calcinated at 500°C for 1 hour.
The synthesized mixed oxide was characterized by Scanning Electron Microscope and Energy Dispersive Spectrometer [Make: FESEM Hitachi SU6600] for quantitative and qualitative analysis. TEM image of nanoparticles synthesized is shown in the Figure 1, which indicates a spherical shape and size in the range 20 to 30 nm. Table 1 shows the elemental composition of mixed oxide nanoparticles, obtained by Energy dispersive spectrum, which confirm the presence of Ce, Zr and O2 in the sample analyzed. XRD patterns for Ce-Zr mixed oxide nanoparticles sythesized by means of co precipitation method is shown in Figure 2.
X-ray diffraction pattern shows the peaks characteristic for fluorite type structures, with planes corresponding to the (1 1 1), (2 0 0), (2 2 0) and (3 1 1).
| Element | Weight% | Atomic% |
|---|---|---|
| O (K) | 22.45 | 68.66 |
| Zr (L) | 22.74 | 12.2 |
| Ce (L) | 54.81 | 19.14 |
| Total | 100 | 100 |
Table 1: Elemental composition of Ce Zr O2 nanoparticle.
Synthesis of Nanofuel
In the present work, two step method has been employed for the synthesis of nanofluids. Cerium zirconium mixed oxide nanoparticles synthesized by co precipitation method was dispersed in various biodiesel blends : B5, B10 and B15, by means of Ultrasonic bath. The agitation time for the synthesis of nanofluids was set as 90 minutes. Oleic acid was added to it as surfactant to improve the stability of nanofluids. Oelic acid is long chain fatty acid, having density higher than that of diesel. In the Ce- Zr-O2 diesel suspension, liphophobic part of oelic acid get attached to the surface of catalytic nanoparticles, thus preventing their agglomeration. The concentration of surfactant was optimized based on the determination of Critical micelle concentration (CMC) which is the concentration of surfactant in the solution above which micelles form and further addition of surfactants added in system goes to micelles .The general way of estimating CMC is by plotting appropriate physicochemical property with respect to surfactant concentration curve and noting the concentration where drastic variation in slope of the plot occurs. In the present work physicochemical property selected for the estimation of CMC was surface tension. The surfactant concentration was varied from 0.01 % vol. to 0.1% vol. in biodiesel blends - B5, B10 and B15 and the surface tension for each concentration was determined by maximum bubble pressure method. In this method the pressure required to force a gas bubble out of a capillary tube, that is vertically immersed in the liquid to be investigated, is determined by increasing the pressure and recording the value of it just before the bubble emerges from the capillary tube into the liquid. The surface tension is calculated from the pressure, radius of the capillary tube and the depth to which it is immersed.
Stabilization of catalytic nanoparticles in diesel - biodiesel blends is one of the major technical challenges in the practical application of cerium zirconium mixed oxide nanoparticles due to chance of agglomeration. Different physical and chemical treatments employed for improving the stability of nanofluids includes addition of surfactant, surface modification of the suspended particles, application of powerful forces on the clustered nanoparticles etc.[28]. The nanoparticle along with surfactant in the biodiesel blends - B5, B10 and B15 were sonicated by means of ultrasonic water bath. The stability of the synthesized nanofluid was determined by zeta potential measurement using Dynamic Light Scattering (DLS) [Make: Malvern Zetasizer Nano] instrument, while varying concentration of Ce-Zr-O2 nanoparticles from 2.5 to 15 ppm. Zeta potential is a measure of the stability of nanofluifs and is the magnitude of the repulsion or attraction between nanoparticles. The zeta potential studies were carried out for different concentrations of nanoparticle at temperatures 35ºC, 25ºC, 15ºC and 5ºC.
Properties of diesel- biodiesel blends such as density, viscosity, flash point, cloud and pour point were determined as per ASTM standards to investigate the effect of the nanoparticles on fuel properties. Density was determined by pycnometer [29] and viscosity was measured using Brookfield rheometer [30]. Flash point of modified fuel was determined by using Cleveland’s flash & fire point apparatus [31] and cloud and pour point was determined by ASTM D97-12 [32].
The effect of nanoparticle dosing in biodiesel blends on performance and emission characteristics was determined by performing constant speed test on a single cylinder four stroke water cooled diesel engine. An Eddy current dynamometer was used for loading the engine and speed was meausured by means of proximity sensor. Various emissions such as NO,HC and CO were measured by means of AVL Emission Analyser. Experiments were carried out by loading the engine from zero to maximum load for different biodiesel blends-B5, B10 and B15 by varying nanoparticle concentration from 0 to 15 ppm. Figure 4 shows the schematic of the engine test rig.
Cerium zirconium mixed oxide nanoparticles, synthesized by co precipitation method, was used as additive, in the diesel - bio diesel blends, in the present work. Biodiesel was synthesized from Jatropha Curcus oil by two step trans esterification process. Zeta potential of catalytic nanoparticles in biodiesel blends was measured by means of DLS. Various properties of biodiesel - diesel blends were determined as per ASTM standards to investigate the effect of the nanoparticles on fuel properties. Engine performance and emission tests were conducted on single cylinder diesel engine and the results were compared.
Oelic acid was used as surfactant in th present work, as it has very low hydrauphilic liphophilic balance (HLB) value. The variation of surface tension of biodiesel blends-B5,B10 and B15 with surfactant concentration is shown in the Figure 3. A sudden drop in the surface tension was observed at the surfactant concentration of 0.05% vol. for all the blends, which corresponds to Critical micelle concentration (CMC) (Figure 3).
The stability of the catalytic nanoparticle added fuel was estimated from Zeta potential measurement, while varying the concentration of nanoparticles and temperature. Temperature based zeta potential analysis shows that zeta potential values increases with increase in temperature. Figures 4a to 4d shows the zeta potential of bio diesel blends at different temperatures. The concentration corresponding to maximum stability at different temperature for B5, B10 and B15 were found to be at 7.5 ppm,10 ppm and 15 ppm respectively, at all temperatures, as shown if Figure 4a-d. B5 biodiesel blend was found to have maximum zeta potential value, hence maximum stability (Figures 4a-4d).
Density
Density of the fuel has great influence on the combustion and engine performance. A nominal increment of 0.58% in the density was observed for B10 biodiesel blends upon nano particles addition as shown in the Figure 5. But there was no significant variation in the density for other blends-B5 and B15 (Figure 5).
Viscosity
Viscosity is the significant property as far as size of fuel droplets and atomization is concerned. High viscosity leads to poor vaporization of fuel spray and extreme pressure in the fuel injection system. Figure 6 shows that there is no significant variation in the dynamic viscosity for B5 and B15 with increase in nanoparticle dosing level. However for B10 biodiesel blend, there is a nominal increment of 1.45% (Figure 6).
Flash Point
Flash point is the highest temperature upto which fuel can be stored, transported and handled safely. An increase in flash point of 5.6% was observed for B5 and B10 blends upon nanoparticle addition as shown in the Figure 7, which point to a decrease in the volatility of the fuel. An increase of 2.7% in flash point was observed for B15 blend. Increase in flash point temperatures is desirable for safe handling of the fuel (Figure 7).
Cloud and pour point
Cloud and pour point indicates the cold behaviour of the fuel. A decrease in pour point by 1ºC was observed for B5 and B10 blends upon 15 ppm nanoparticle addition as shown in the Figure 8,whereas there was no variation was observed for B15 blend. The decrease in the pour point may be due to delay in the formation of hydrocarbon clusters with nanoparticle addition, upon cooling (Figure 8).
Performance and Emissions studies
Brake thermal efficiency
Figures 9a to 9c shows the variation of brake thermal efficiency with the concentration of catalytic nanoparticle for B5,B10 and B15 blends. An enhancement of 8.7% and 15.3% in brake thermal efficiency was observed for B5 and B10 blend respectively with 15 ppm nanoparticle addition. This may be attributed to the catalytic effect and oxygen buffering capability of cerium zirconium oxide nanoparticles present in the fuel which promote longer and more complete combustion. Improvement in the brake thermal efficiency with blending may also be due to increase in lubricity of biodiesel which might reduce the friction loss [33] (Figures 9a-9c).
NO Emission
Figures 10a to 10c shows the variation of NO emission with nanoparticle dosing for B5, B10 and B15 blends. NOx formation mainly depends on two factors, namely, oxygen availability and the combustion temperature. A slight increase in NO emission was observed for pure B5 blends, which may due to higher oxygen content associated with biodiesel blends, as compared to diesel. NO formation was found to increase with load, which may be due to increase in the combustion temperature at higher loads. An overall reduction in NO emission was observed for biodiesel blends with the addition of catalytic nanoparticle in diesel bio diesel blends. 22.8% reduction of NO emissions was observed at 3/4th load for B5 blend for catalytic nanoparticle concentration of 10 ppm. Similarly maximum average reduction of 15 % was observed for B5 and B10 blends for catalytic nanoparticle concentration of 15 ppm. This reduction in NO emissions may be attributed to dual valence states of Ceria. Cerium oxide (CeO2) supplies the oxygen for the reduction of the hydrocarbon as well as the soot, and gets converted to Cerous oxide (Ce2O2), which in turn will re-oxidized to CeO2 through the reduction of nitrogen oxide, as per following reactions
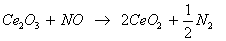 (2)
(2)
HC Emissions
Figures 11a to 11c shows variation of HC emission with concentration of nanoparticle for B5, B10 and B15 blends. HC emission was found to higher at lower loads which may be due to lower heating value of biodiesel which necessitate high local fuel-air ratio resulting in the increase of HC emissions [34]. 24% reduction in HC emissions was observed at half load for B5 blend with 10 ppm nanoparticle dosing and 25% reduction was observed for B15 with 15 ppm nanoparticle dosing. Reduction in HC emissions may be due to redox nature of Ce-Zr-O2 nanoparticles which cause oxidation of hydrocarbons. The mixed oxide nanoparticle also lowers the soot oxidation temperature and thus enhances hydrocarbon oxidation, promoting complete combustion (Figures 11a-11c).
Use of fuel born catalyst, Cerium zirconium oxide nanoparticles cause reduction of harmful diesel engine emissions due to its peculiar redox functionality and oxygen buffering capability. Zirconium doped cerium oxide nanoparticles was synthesized by co precipitation method. TEM image confirms that synthesized nanoparticles are in the size range of 20 - 30 nm. Stability of the nanoparticles in fuel was improved by the addition of Oleic acid. Surfactant concentration was optimized and found to be 0.05% vol., by Critical Micelle Concentration (CMC) test based on measurement of surface tension. Ce- Zr-O2/biodiesel blends nanofluids were synthesized using ultrasonic shaker by two step method. The stability of nanofluids was determined by Zeta potential measurement. The concentration corresponding to maximum stability at different temperatures for B5,B10 and B15 blends were found to be at 7.5 ppm, 10 ppm and 15 ppm respectively. No significant change in the properties was observed on the addition of Zzirconium doped cerium oxide nanoparticles in diesel- biodiesel blends. The mixed oxide nanoparticle was found to be effective in reducing NO emission with biodiesel blends. 15% average reduction of NO emissions was observed for B5 and B10 blends with 15 ppm of catalytic nanoparticle concentration. A maximum reduction of 25% was observed in HC emission for B15 blend with 15 ppm catalytic nanoparticle concentration. Ce- Zr-O2 dispersed in the biodiesel blends along with surfactant was found to be effective in enhancing engine performance and reducing harmful emissions.
Authors greatly acknowledge the support from Hindustan Petroleum Corporation limited (HPCL), India.