Keywords
|
| Point-resolved spectroscopy (PRESS), CSD correction, Error-Map, Blind Source Separation |
INTRODUCTION
|
| The adult body normally forms new cells only when they are needed to replace old or damaged ones. Infants and children create new cells to complete their development. A tumor develops if normal or abnormal cells multiply when they are not needed. The necrosis is the death of the body cells. A brain tumor is a mass of unnecessary cells growing in the brain or central spine canal. The major types of brain are primary and metastatic brain tumors.Primary brain tumors start and tend to stay, in the brain. Metastatic brain tumors originate as cancer somewhere in our body and spread to the brain. The primarybrain tumors are further classified into benign brain tumors and malignant brain tumors.Benign brain tumors are a slow growing cell which contains distinct borders and they rarely spread. Malignant brain tumors are usually rapid growing cells, and are invasive. Grade I tumors are the least malignant and are usually associated |
| Grade I tumors are the least malignant and are usually associated with long-term survival. Grade II tumors are relatively slow-growing and have a slightly abnormal microscopic appearance. Grade III tumors are actively reproducing abnormal cells which grow into nearby normal brain tissue. Grade IV tumors reproduce rapidly and they form new blood vessels to maintain their rapid growth. |
| DESI technique identifies the cancer type, grade and a tumor margin in faster manner.LC model is fully automatic and non-interactive tool which automatically quantifies the in vivo proton MR spectra. Atomic force microscopy (AFM) is a very high-resolution type of scanning probe microscopy, which is useful for imaging. An MRI scan offers brain images with excellent anatomical detail that provides clarity of the small structures in the brain, but the images often lack quantitative or finely measurable, information. (MRS)Magnetic Resonance Spectroscopy produces images depicting function rather than shape. The equipment requires a special, highly complex facility. MRSI (Magnetic Resonance Spectroscopic Imaging) scan, which provides both the spatial information and also the tumor details along with tumor grade. |
| CT uses ionizing radiation but MRI uses strong magnetic field to align the nuclear magnetization then radio frequencies changes the alignment of the magnetization which can be detected by the scanner. That signal can be further processed to create the extra information of the body. The proposed system is based on unsupervised method for creating nosologic images using the MRSI data. It provides mixture of primary colors (red, green, blue) for each tissue patterns (normal, tumor and necrosis). |
| In this paper, we first start with the data preprocessing for noise removal and then continues with the process of tissue differentiation that founds out the different tissues that are present in the given sample image.k means clustering is also used for the calculation of distance. CSD correction is performed to remove the artifacts of chemical shift displacement and the lipid contamination. The non-informative cells that are usually present at the borders are being removed to ensure the spectral quality. Thenosologic images are obtained that gives the overview about the heterogeneity of the tissues. Finally the reliability of the investigation can be achieved using the error map calculation. |
RELATED WORK
|
| In [4] pattern recognition techniques were used to segment the MRSI data. Twosteps CCA technique is used to identify the different tumor types (tumor typing step and segmentation step).CCA method translates short echo-time brain MRS images into easy-to-understand nosologicimages. So it distinguishes different brain tumor type’s reliably.MRSI and MR image information is integrated to improve the performance. The main advantage of this work was that the nosologic images can be easily understood. In[5] the authors have used cNMFwhich automatically and simultaneously obtain the tissue patterns and also achieves tissue segmentation.It performs iterative selection of the data. This ensures fast recovery of biochemically meaningful spectral sources. The benefit of this work is that itautomatically identifies tissue patterns and corrects the distortions.It also measures a variety of neurological disorders. In [6] CCA was used which gives spectral and spatial information simultaneously. CCA was applied on the 2DTSI for identifying heterogeneous tumors effectively. But it gives poor performance in the mixed tissue regions. Authors have compared the CCA with OCA and obtains high performance with CCA.In [7] authors have used Hierarchial nonnegative matrix factorization, whichcan accurately determines three tissue patterns. The spatial distributions are also estimated using non-negative least square estimation. Data preprocessing was done to remove the residual water components.hNMFcan also be applied for high grade brain tumors.It provides guidance for surgery or radiotherapy. In [8]Combined MRI and MRSI was used to create nosologic imaging.First the segmentation is performed and the tumors gets splits into normal and abnormal. The abnormal tissue is classified into different tumors based on supervised methods. In this work, asingle nosologic image gives information about different tissue patterns and also hhigh resolution images can be created. |
PROBLEM STATEMENT
|
| The existing system used supervised methods for the diagnosis of brain tumor. This obviously requires a lot amount of training data sets. This also requires large training classifiers. The time taken for the analysis or comparison of data with the training data sets is also high.The existing system used only two colors for normal and abnormal tissues. So the heterogeneity of tissues cannot be identified. If the classified abnormal tissue is further grown or subdivided into tumoror necrosis, then those multiplications of tissues cannot be identified. The NMR scans are used for the scanning of brain tissues. |
| A. Canonical Correlation Analysis(CCA) |
| CCA is a statistical methodthat can beable to concurrently discover the spectral and spatial information illustrating the MRSI data. Here, the performance ofCCA is additionallyexamined by using brain data obtained by twodimensional turbo spectroscopic imaging (2DTSI) frompatients affected by glioblastoma. The purpose is to investigate the applicability of CCAwhen typing tissues ofheterogeneous tumors. The performance of CCA is also compared with ordinary correlation analysis. The results show that CCA outperforms ordinary correlation analysis in terms of robustness andaccuracy.CCA founds out the relationship between two sets of variables. CCA is useful for identifying five tissue regions [6]. |
| B. Drawbacks of CCA |
| • CCA is complex to the shape of the model spectra. |
| • It gives poor performance in the mixed tissue regions compared to the other tissue regions. |
| • Quantifying the degree of uncertainty is an open problem. |
PROPOSED METHODOLOGY
|
| With this proposed methodology, we are focussing fully on unsupervised method based on blind source separation specifically on nonnegative matrix factorization (NMF) [1] to automatically create nosologic imagesof gliom’s. Hierarchical tissue pattern differentiation method using NMF (hNMF) is able to differentiate three tissue patterns present in glioblastomamultiforme (GBM,or grade IV glioma).Image preprocessing should be done as a first step in order to remove the residual water components. This step is called as the pre-processing step which can be done using a method called as HLSVD-PRO (Hankel–Lanczos singular value decomposition)[7].The in-house software SPID is used for this method. Tissue differentiation is performed then to differentiate the tissues as normal and abnormal. An optimal threshold value is set to differentiate the abnormal tissue. In addition with the existing techniques, we are using a method called as The CSD (Chemical Shift Displacement) correction eliminates the borders and we can get effective spectral quality. Finally the nosologic images are created. Error-maps are also calculated for each nosologic image for reliability investigation. |
| The main objective of this paper is propose a method that ensures sufficient spectral quality and to give a better tissue differentiation.it should also reduce the number of training sets for creating the nosologic imaging using unsupervised method. The time consumption for creating the nosologic image is being reduced. |
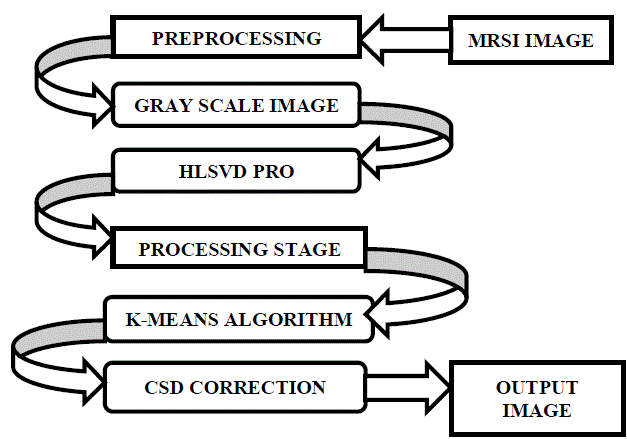
| A. Flow Diagram for proposed methodology |
| B. Preprocessing |
| After attaining the images of brain, the process of preprocessing should be done to remove the noise and the unwanted residual water components from the sample. The image is first reshaped to our convenient of 256×256, and then the following steps are being processed. Some spectra of insufficient quality are excluded, where they are usually present at the borders. The post processing should also be done using eddy current technique. Eddy current correction contains gradient coils which creates MR images. The eddy current is the coil that is useful for imposing the shears and stresses. As a result, we can get only the necessary components inside the brain samples. HLSVD-PRO removes the residual water components using SPID toolbox [7]. Negative values in the spectra were set to 0 to ensure the nonnegativity as they were caused by the noise. |
| C. Tissue Differentiation |
| After the preprocessing is done, the tissues are differentiated into normal and the abnormal tissues. K-means clustering algorithm is being used for identifying the different tissue patterns. This algorithm forms clusters of similar items. Tissues of similar intensities are grouped into one cluster and the dissimilar tissues are formed into another cluster. At a maximum of five clusters can be formed and the necessary information alone can be obtained.k-means algorithm works by the distance calculation. Color map is useful for achieving different colors for the tissues that are obtained from the MRSI scan. |
| 1) Hierarchical Non Negative Matrix Factorization (hNMF): An hNMFfirst separates the brain tissue into normal and abnormal, and then by applying an optimized threshold, the abnormal tissue is further separated into tumor and necrosis. Non-negative matrix factorization founds out only two tissue patterns at each level. So we are using hNMF which can discover three tissue patterns. The three most significant spectral sources for GBMs are recovered. The spatial information of the brain sample can also be obtained. The absence or presence of necrosis only decides whether two or three spectral sources are more appropriate for each MRSI dataset. |
| D. Chemical Shift Displacement Correction(CSD) |
| Here, we propose an approach to take the CSD artifact into account when evaluating the spatial distribution of tissue-specific spectral sources obtained from hierarchical non-negative matrix factorization. |
| 1) Spectral Sources Estimation:X is approximately factorized as the product X = WH using a hierarchical nonnegative matrix factorization algorithm. The k columns of W (k=2 or 3) are called spectral sources and represent the most distinct tissue-specific spectra within the considered MRSI dataset. The k rows of H encode the linear mixing weights of each spectral source for each spatial location in the cropped MRSI matrix. |
| 2) Spatial Re-Estimation with CSD Correction:Spatial weights for each spectral source are computed from all voxels in the non-cropped MRSI matrix as follows: a non-negative least squares (NNLS) problem is solved for each voxel, in order to express each spectrum as a linear combination of the already computed k spectral sources in W. However, the columns of W are first filtered in a location-dependent way, in order to approximate the effect of the CSD artifact at each location. |
| E. Nosologic Imaging |
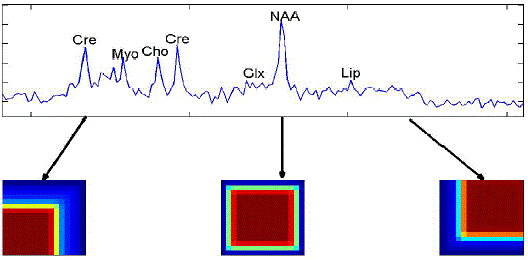
| A single nosologic imaging can give different tissue patterns and tumor infiltration. We can easily get all the information regarding the tumor type, grade and tumor concentration.hNMF method recovered two spectral sources for low grade glioma patients which does not spread too much faster. We can easily get three spectral sources were computed for highest grade gliomas using this nosologic imaging. The intensity of the color in the tumor obtained shows the percentage of tumor that is present in the brain of the patient. If the colors are dark; it shows the aggressiveness of tumor. Lower reliability at voxels is usually present on the outer borders compared to inner voxels [4]. |
| Nosologic image summarizes all the accessible information by coloring each pixel according to the determined histopathological class.Nosologic images can be regularly and easily be used by radiologists for tumor diagnosis [8]. |
| Unsupervised nosologic images, created by encoding the weighting matrices corresponding to each spectral source as a channel in an RGB image, exhibit black borders if CSD correction is not applied, meaning that all spectral sources have weight close to zero in those regions. This effect practically disappears when CSD correction is applied, although estimated error maps (data not shown) indicate lower reliability at voxels on the outer borders compared to inner voxels. |
| F. Calculation of Reliability for Nosologic Images |
| For the least squares problem in |
| Min||xi-Whi|| over hi≥ 0, hi € ?r |
| If W is estimated in a correct way, we can almost guess lower bounds for the standard errors of the linear combination weights hi at the ith voxel as the diagonal elements of the inverse Fisher information matrix σ2i(WTW)-1.The residual error ||xi –W hi|| divided by the degrees of freedom can be used to estimate σ2i ,which is an estimate for the noise variance for the spectrum in voxel i [1]. |
CONCLUSION
|
| Unsupervised nosologic imaging provides a novel way for MRSI data interpretation without the need of large training datasets. The created nosologic image is obtained on the whole PRESS excitation volume and mixed tissues in heterogeneous tumors can be shown as mixtures of primary colors. In this, the chemical shift displacement artifact has been taken into account for re-estimating the spatial distribution of tissue-specific spectral sources extracted from glioma patients using a rapid short echo time 2D MRSI acquisition protocol based on the PRESS volume localization method. This permits recovery of tissue distribution closer to the borders of the PRESS volume. The data preprocessing was done to remove the residual water components and it eliminates the low quality spectra. This can be done by using the HLSVD-PRO which uses the in-house software SPID.The CSD correction removes the non-informative cells which are present at the borders of the PRESS excitation volume. This ensures better tissue differentiation. |
| |
Figures at a glance
|
 |
 |
| Figure 1 |
Figure 2 |
|
| |
References
|
- Yuqian Li, Diana M. Sima, Sofie Van Cauter, UweHimmelreich, Anca R. CroitorSava,Yiming Pi, YipengLiu, and Sabine Van Huffel,”Unsupervised NosologicImagingforGlioma Diagnosis” ,IEEE transactions on biomedical engineering, vol. 60, Issue 6,pp. 1760-1763,2013
- Daniel D. Lee,H. Sebastian Seung,” Learning the parts of objects by non-negative matrix factorization”, Nature,vol. 401,pp.788-791,1999
- P. Sajda, S. Du, T. R. Brown, R. Stoyanova, D. C. Shungu, X. Mao, and L. C. Parra, “Nonnegative matrix factorization for rapid recovery ofconstituent spectra in magnetic resonance chemical shift imaging of the brain,”IEEE Transaction on medical images, vol. 23, Issue. 12, pp.1453–1465,2004
- M. De Vos, T. Laudadio, A. W. Simonetti, A. Heerschap, and S. Van Huffel, “Fast nosologic imaging of the brain”, J. Magn. Reson, vol. 184,Isssue 2, pp. 292–301,2007
- S. Du, X. Mao, P. Sajda, and D. C. Shungu, “Automated tissue segmentation and blind recovery of 1 H MRS imaging spectral patterns ofnormal and diseased human brain”,NMR Biomed., vol. 21, pp. 33–41, 2008.
- T. Laudadio, M. C. Martinez-Bisbal, B. Celda, and S. Van Huffel, “Fast nosological imaging using canonical correlation analysis of brain dataobtained by two-dimensional turbo spectroscopic imaging,” NMR Biomed., vol. 21, Issue 4, pp. 311–321.2008
- Y. Li, D. M. Sima, S. Van Cauter, A. Croitor Sava, U. Himmelreich, Y. Pi, and S. Van Huffel, “Hierarchical non-negative matrix factorization(hNMF): A tissue pattern differentiation method for glioblastomamultiforme diagnosis using MRSI,” NMR Biomed, to be published.
- J. Luts, T. Laudadio, A. J. Idema, A. W. Simonetti, A. Heerschap, D. Vandermeulen, J. A. K. Suykens, and S. Van Huffel, “Nosologic imagingof the brain: segmentation and classification using MRI and MRSI,” NMR Biomed., vol. 22, Issue. 4, pp. 374–90, 2009.
|