Keywords
|
| Electrical conductivity, thermal stability, organic/organic composite, polyacrylonitrile/polypyrrole |
INTRODUCTION
|
| Conducting polymers are polymeric materials with metallic and semiconductor characteristics, a combination of properties not exhibited by any other known material. A key property of conductive polymers is the presence of conjugated double bonds along the backbone of the polymer. The chemistry noble prize in 2000 was awarded for the discovery and study of conducting polymers to the three scientists namely: Alan G.Mac Diarmid, Alan J. Heeger and Hideki Shirakawa. The biggest advantage of conductive polymers is their processability by dispersion. Conductive polymers combine the mechanical properties (flexibility, toughness, malleability, elasticity etc.) of plastics with high electrical conductivity. These properties can be fine tuned using different methods of organic synthesis. Conducting polymers have potential applications in various fields. The conductive and semiconducting properties of these polymers make them an important class of materials for a wide range of electronic, optoelectronic and biotechnological applications such as in rechargeable batteries, molecular electronics, electronic displays, solar cells, ion exchange membrane in fuel cells, diodes, capacitors, field-effect-transistors, printed circuit boards, chemical sensors, drug release systems and biosensors, etc. [1–4]. It is being projected that conducting polymers can be used to transport small electronic signals in the body, i.e. act as artificial nerves. Although conducting polymers have various potential applications there are some of the factors which hinders some of their applications such as the cyclability of conducting polymer is poor because electrochemical degradation occurs on the polymers leading to the decrease in pseudo capacitance and conductivity, lack of solubility, brittleness and lack of long term electrical stability further restricts their applications. To overcome these issues much efforts and significant improvements have been made to raise their applications. This can be done by using conducting polymer composites. Composites of intrinsically conducting polymers (ICPs) are materials that utilize conjugated polymers and at least one secondary component that can be inorganic or organic materials or biologically active species. The goal is to produce a new composite material that has distinct properties that were not observed in the individual components. This may include either new or improved chemical properties that can be exploited for chemical or biological sensing. |
| CPCs are obtained by blending insulating polymers with conductive fillers such as carbon black, carbon fibres, metal particles or conducting polymers such as polyaniline [5]. Aim of using composites is to make use of the merits of the two materials. Conducting polymer composites are currently gaining attention for technical applications. Combined mechanical, thermal, and electrical interaction between the filler particles via their electrical contacts and the surrounding polymer host matrix are responsible for the properties of the composite material. Different types of conducting polymer composites have been used and find applications in various fields. There are several advantages of conducting polymer composites over polymers such as high conductivity, low density and low weight, high mechanical characteristics, enhanced resistance to humidity, low sensing temperatures, low cost and possible to process by highly productive industrial methods. The major advantage of conducting polymers composite materials over the polymers alone is based on the increase in active surface area and ability to form good electronic contact between the composite components and the transducer. The parent polymer provides high dispersion and high surface area for the secondary components to be integrated and creates templates for chemical reactions and interactions. The inherent stability and symbiosis between the two components used to create the composite material is often superior to the bulk components alone. Composites combine both the characteristics of polymer and metal or carbon. Electrical properties can be close to metals while the processing is typical for the polymers. Conducting polymers have potential applications at all levels of microelectronics like electrostatic discharge (ESD), electromagnetic interference (EMI) shielding, interconnection technologies, corrosion protection of metals, and in devices like diodes, transistors, sensors, biosensors, and actuators [6-10]. In addition, polymeric materials are light weight, flexible, and can be easily processed which makes them suitable for micro and nano scale molecular electronic devices. Also, they find application in the detection of single molecule, thus creating future opportunities for high sensitivity sensors and biosensors. In this study, PAN/Ppy composites were prepared by sol-gel method and studied for the determination of electrical conductivity by using fourin- line probe dc electrical conductivity measurement instrument. |
MATERIALS AND METHODS
|
| The reagents used for the synthesis of the material were obtained from CDH, Loba Chemie, E- Merck and Qualigens (India Ltd.). All other reagents and chemicals were of analytical reagent grade. A four-in-line probe electrical conductivity-measuring instrument, Scientific Equipment (India), was used for measuring the dc electrical conductivity. A hydraulic pressure instrument was used for making pellets of sample materials. An electronic balance (digital), Sartorius (Japan), model 21 OS was used for weighing purpose. |
PREPARATION OF COMPOSITE MATERIAL
|
| Various samples of polyacrylonitrile/polypyrrole (PAN/Ppy) were prepared by mixing one volume of 5 weight% solution of polyacrylonitrile solution with different volume of pyrrole (approximately 3.33 wt% solutions in toluene) as given in Table 1. FeCl3 solution (0.1 M) was mixed thoroughly with the PAN/Pyrrole, continuous stirring was done during the addition of FeCl3 solution, slowly the white gel of precipitate gel PAN/Ppy turned first to green and then to black. The reaction mixture was kept for 24 h under ambient condition (25 ± 2 °C). The reaction mixture was then kept for 24 h under ambient conditions (25 ± 2 °C). Then the polypyrrole based composite gel was filtered off, washed with 0.75 M HCl and then washed thoroughly with DMW to remove excess acids and any adhering traces of ferric chloride. After filtration the gel was dried at 50 °C in an air oven for 48 h. The dry product was then crushed into small granules when immersed in DMW. The material was again washed with acetone in a soxhlet, finally dried at 50 °C and kept in a desiccator. Hence a number of composite samples of PAN/Ppy were prepared. |
ELECTRICAL CONDUCTIVITY MEASUREMENTS
|
| The composite samples were treated with 0.5 M aqueous solution of HCl and washed repeatedly with DMW to remove excess HCl until the filtrates gave a negative test for hydrogen ions. The sample materials were dried completely at 50 °C in the oven. Then 0.5 g material from each sample was finely ground in a mortar pastel and pellets were made at room temperature with the help of a hydraulic pressure instrument at 25 KN pressure for 20 min. The thickness of each pellet was measured by a micrometer. Four-probe electrical conductivity measurements with increasing temperatures (between 35 and 200 °C) for the composite samples were performed on pressed pellets by using a 4-in-line-probe dc electrical conductivity-measuring technique. The sample to be tested was placed on the base plate of the four-probe arrangement and the probes were allowed to rest in the middle of the sample. A very gentle pressure was applied on the probes and then it was tightened in this position so as to avoid piercing the samples by the probes. The arrangement was placed in the oven. The current was passed through the two outer probes and the floating potential across the inner pair of probes was measured. The oven supply was then switched on, the temperature was allowed to increase gradually, and the current and voltage were recorded with rise in temperature. |
| Table 1 Conditions of the preparation of PAN/Ppy composite |
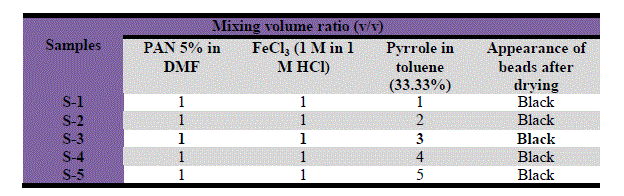 |
RESULTS AND DISCUSSION
|
| In this study, various samples of electrically conducting composite materials were prepared by the incorporation of polypyrole in to the matrices of PAN by mixing different concentrations (vol. %) of organic monomers pyrrole into the fixed volume of PAN solution. The composite material is basically the mixture of two polymers i.e. polypyrrole and PAN. The organic polymer PAN of the composites is the insulator while polypyrrole is the good electronically conducting polymers. In general, a high electrical conductivity of conductive polymers is attained by dopant, which stabilizes the polaron and bipolaron states as counter anions [11-14]. It is well understood that the dc electrical conductivity of composite materials is due to the presence of sufficient amount of the conducting polymer and basically it is electronic conduction contributed by the conducting components, i.e. polypyrrole by the charge-transfer reaction between the conducting component i.e. polypyrrole and doping agents, HCl, as given: |
| [Polypyrrole/PAN] + n HCl → [Polypyrrole (n H+)(n Cl-)/PAN] |
| The current-voltage data observed by a 4-in-line probe dc electrical conductivity-measuring instrument was processed for calculation of resistivity (ρο) using the following equation- |
| ρÃÂþ = (V/I) × 2 πS |
| where V is the voltage (V) and I is the current (A). |
| Since the thickness of the sample is small compared to the probe distance a correction factor for it has to be applied and the corrected resistivity may be calculated as- |
| ρ = ρο/G7 (W/S) |
| where ρ is the corrected resistivity in ohm. cm., G7(W/S) is the correction factor used in the case of non-conducting bottom surface and it is a function of W, thickness of the sample under test (cm) and S, probe spacing (cm); i.e., |
| G7 (W/S) = (2S/W) loge2 |
| Thus, the electrical conductivity (σ) was calculated using the following equation- |
| σ = 1/ρ |
| where σ is the electrical conductivity in Scm-1. |
| The dependence of the electrical conductivity through the bi-phasic systems i.e. PAN/Ppy prepared with different volume% of pyrrole was examined (Table 2). A slight increase in the electrical conductivity for of this composite is followed at a certain pyrrole concentration by a sudden jump, which is again followed by moderate increase (Table 2). At about 1:1:3 mixing volume ratio of PAN/FeCl3/pyrrole (critical concentration of conducting phase), the sharp rise in electrical conductivity is observed that could possibly be explained on the basis of percolation theory [15]. It is clear that the main force to towards the electrically conductivity of the composite material is the presence of conducting polymer i.e. polypyrrole. |
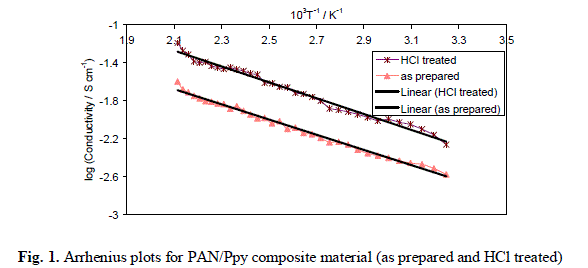
| The variations of electrical conductivity (σ) of PAN/Ppy composite samples (as prepared and HCl treated), prepared by 1:1:3 mixing volume ratio of PAN/FeCl3/pyrrole (S-3) with increasing temperature (between 35 °C to 200 °C) was carried out. On examination, it was observed that the electrical conductivity of the composite samples increases with the increase in temperature and the values lie in the order of 10-4 to 10-2 S cm-1 i.e., in the semiconductor region. To determine the nature of dependence of electrical conductivity on temperature plots of log σ versus 103T-1 K-1 was drawn (Fig. 1). It was observed that the composite followed Arrhenius equation similar to other semiconductors [16]. It was also observed that the composite material showed enhanced electrical conductivity on exposure to HCl as compared to original form, due to the charge-transfer reaction between polypyrrole component of the composites and doping agents, HCl as described above. |
 |
 |
| The thermal stability of the PAN/Ppy composite materials (HCl treated) in terms of dc electrical conductivity retention was studied under isothermal condition (at 50, 70, 90, 110, 130 and 150 °C) measuring 4-in-line- probe dc electrical conductivity at an interval of 15 minutes. The electrical conductivity measured with respect to the time of accelerated ageing is presented in Fig. 2. |
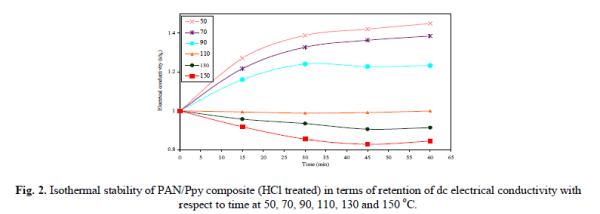 |
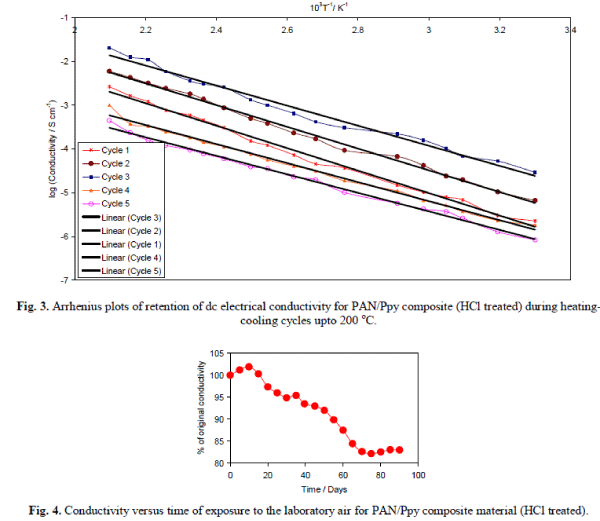
| It was observed that the electrical conductivity for the composite materials is quite stable at 50, 70, 90 and 110 °C that support the fact that the dc electrical conductivity of the composites is sufficiently stable under ambient temperature conditions. The electrical conductivity decreases with time at 130 and 150 °C that may be attributed to the loss of dopant and the chemical reaction of dopant with the material. The stability of HCl treated composite material in terms of electrical conductivity retention was also monitored for 5 cycles by repeatedly measuring linear four-probe dc electrical conductivity with increase in temperature at an interval of 45 minutes and the dc conductivity for each heating cycle was plotted as log σ versus 103T-1K-1 as shown in Fig. 3. It was observed that the PAN/Ppy composite materials followed the Arrhenius equation for its temperature dependence similar to other semiconductors [17]. There was minor difference in their electrical conductivity even after repeating the experiment for five times that showed the good stability of the material during heating-cooling cycles under severe oxidizing conditions upto 200 °C. PAN/Ppy was also observed to be a stable material, i.e., the room temperature conductivity is negligibly affected by short-term exposure to laboratory air as evident from Fig. 4. |
 |
CONCLUSION
|
| Polyacrylonitrile/polypyrrole composite showed the enhanced conductivity in doped form. It was also observed that the conducting nature depends on the percolation behavior of conducting phase. The nature of dependence of electrical conductivity with rise in temperature showed that the composite followed Arrhenius equation similar to other semiconductors. The composite material was also found thermally stable for cyclic and isothermal studies. It was also found environmentally stable electrically conducting polymer. |
ACKNOWLEDGEMENTS
|
| The authors are thankful to the Department of Electrical Engineering, Sri Vankateshwar University and Aligarh Muslim University, for providing necessary research facilities. |
| |
References
|
- Diaz, A. F., Kanazawa, K. K., Gardini. G. P., Electrochemical polymerization of pyrrole, J. Chem. Soc. Chem. Commun., vol.14, pp.635-636,1979.
- Saraswathi, R., Gerard, M., Malhotra. B. D., Characteristics of Aqueous Polycarbazole Batteries, Journal of Applied Polymer Science, vol.74,pp.145-150, 1999.
- Kawai, T., Kuwabara, T., Wang, S., Yoshino. K., Secondary Battery Characteristics of Poly(3-alkylthiophene), Japanese Journal of Applied Physics, vol.29, pp.602-605, 1990.
- Weetall, H. H., Druzhko, A., RdeLera, A., Alvarez, R., Robertson. B., Measurement of proton release and uptake by analogs ofbacteriorhodopsin. Bioelectrochemistry, vol.51, pp. 27-33, 2000.
- Narkis, M., Zilberman, M., Siegmann. A., “On the curiosity of electrically conductive melt processed doped polyaniline/polymer blends versuscarbon black /polymer compounds Polymers for advanced technologies”, vol.11, pp. 101-110, 2000.
- Kraft, A., Grimsdale, A. C., Holmes. A. B., “Electroluminescent conjugated polymers-seeing polymers in a new light”, Angew. Chem. Int.Edit., vol.33, pp. 402-428, 2000.
- Hepburn, A. R., Marshall, J. M., Maud. J. M., “Novel electrochromic films via anodic oxidation of carbazolyl substituted polysilaxones”, Synt.Met., vol.43, pp. 2935-2938, 1991.
- Dubois, J. C., Sagnes, O., Henry. F., “Polyheterocyclic conducting polymers and composites derivatives”, Synt.Met., vol. 28(1-2), pp. C871-C878, 1989.
- Roncali, J., Garreau, R., Delabouglise, D., Garnier, F., Lemaire. M., “Communications modification of the structure and electrochemicalproperties of poly (thiophene) by ether groups”, J. Chem. Soc. Chem., vol.11, pp. 679-781, 1989.
- Burke. A., “Ultracapacitors: why, how, and where is the technology”, J. Power Sources, vol.91, pp.37-50, 2000.
- Murakami, A., Hirose, M., Terashima, M., Kuze, K., Sakaizumi, T., Ohashi, O., Mol. Cryst. Liq. Cryst., vol. 224, pp. 61-72, 1993.
- Thieblemont, J. C., Brun, A., Marty, J., Planche M. F., Calo, P., Thermal analysis of polypyrrole oxidation in air, Polymer, vol.36,pp.1605-1610, 1995.
- Khan, A.A, Inamuddin, Cation-exchange kinetics and electrical conductivity measurement studies of an electrically conducting ‘organicinorganic’compositecation-exchanger: polypyrroleTh(IV) phosphate, J. Appl. Polym. Sci. vol 105, pp 2806-2815, 2007.
- Thieblemont, J. C., Planche, M. F., Petrescu, C., Bouvier, J. M., Bidan, G., Stability of chemically synthesized polypyrrole films, Synth. Met.,vol.59, pp.81-96, 1993.
- Stauffer, D., “Introduction to percolation theory”, Taylor and Francis, London, (1985).
- Mohammad, F., “Handbook of Advanced Electronic and Photonic Materials and Devices”, Nalwa, H. S., (Ed.), Academic Press, New York,(2000) 321.
- Khan, A.A., Alam, M.M., Inamuddin, Mohammad, F., Electrical conductivity and ion-exchange kinetic studies of a crystalline type ‘organicinorganic’cation-exchange material: polypyrrole/polyantimonic acid composite system, (Sb2O5) (-C4H2 NH-). nH2O, J. Electroanal.Chem. Vol572 pp 67-78, 2004.
|