ISSN: 2320-0189
ISSN: 2320-0189
Ritu Shukla*, Dinesh C Sharma, Neelam Pathak and Priti Bajpai
Department of Biosciences, Integral University, Lucknow 226026, India
Received date: 20/06/2016 Accepted date: 29/08/2016 Published date: 04/09/2016
Visit for more related articles at Research & Reviews: Journal of Botanical Sciences
Grewia asiatica Linn. (Grewoideae) has been recognized as a traditional plant for the curing of various human disorders. The aim of present study was to evaluate different extracts (n-hexane, dichloromethane, methanol and aqueous) of fruit for estimation of phytoconstituents and antioxidant activity. Our results illustrated that methanol fruit extract exhibited strong antioxidant potential by DPPH (1, 1-diphenyl- 2- picrylhydrazyl) 90.1% (IC50-62.75 ± 0.069 μg), ABTS (2,2'-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) 65.98% (224 ± 0.032 μg), superoxide 71.1% (IC50-53.6 ± 0.05 μg) and FRAP (Ferric Reducing Antioxidant Potential) assay 7.68 ± 0.04 μmole Fe(II)/mg) generated free radicals. Furthermore, linear correlation studies have been performed between total phenolic content, total flavonoid content and FRAP assay, which determine the regression coefficient as 0.827 and 0.956 (respectively for total phenolic content and total flavonoid content). Moreover, methanol extract exhibited significant OH radical scavenging 91.1% (IC50 43.80 ± 0.03 μg). Altogether, the present study revealed methanol fruit extract as a potential source of antioxidants used in an ethno-medical system, explanatory for therapeutic application of free-radical-induced diseases. Methanol extract was also identified as 2-furancarboxaldehyde, 5-(hydroxymethyl); 2,3-dihydro-3,5- dihydroxy-6-methyl-4h-pyran; Cis-vaccenic acid majorly by Gas Chromatography- Mass Spectrometry analysis.
Grewia asiatica, Total phenolic content, Total flavonoid content, Ethanomedical
Plant products are non-toxic, have no adverse effects with well-organized, protective as well as curing property which is now a day’s center attraction for the researchers to develop new scaffold of the drug. Since ancient times, Medicinal plants have been practiced to cure the diseases and for the good health of humankind [1-3]. The diversity of drugs of modern pharmacology is developed from plant origin compounds [4]. These Compounds are the marvelous resource to develop new drugs and demonstrate plentiful bioactivities as antioxidant compounds that are used in food as well as in pharma industries [5,6]. Excessive generations of free radicals are the cause of oxidative stress, which plays an important role in several diseases [7].
Free radicals such as hydroxyl radicals, superoxide anion and H2O2 are reactive oxygen species, which is responsible for the stimulation of oxidative stress and caused lipid peroxidation, damage to a wide range of other biomolecules through a process that is supposed to be implicated in the etiology of various diseases such as diabetes, heart diseases, stroke, rheumatoid arthritis and cancer [8-11]. Antioxidant plays a key role in scavenging free radicals, thus providing protection to human beings against infections and degenerative diseases however, recent concern has been principal regarding for the determination of unusual side effects of synthetic one in human beings [8,12,13]. The two most commonly known synthetic antioxidants (butylated hydroxyanisole and butylated hydroxytoluene) have not been in practice due to their toxic effects [14,15]. Moreover, synthetic drugs frequently have side effects], whereas herbal formulations are comparatively much safer in this respect. Widespread ethnobotanical examination has projected on the detection of important formulations during the past few decades [16,17]. Traditional medicinal plants are normally available, economically favorable and delicate replacement of synthetic medicines. Approximately 60% to 80% of the world’s population still depends on traditional formulations for curing of general ailments [18]. Antioxidant compounds are neutralizing free radicals, which will be helpful for humanity in the development of new therapeutic natural compounds from medicinal plants [19]. These bioactive compounds are of various classes of phytocompounds such as flavonoids, saponins, steroids, alkaloids, terpenoids, polysaccharides and tannins that helped in the various bio-actions in ancient and recent curing methods [20,21]. The traditional treatment system Ayurveda mentions many herbal drugs for the curing of various diseases. Grewia asiatica is an important medicinal plant of Grewoideae family, which is widely used in traditional curing [22]. In the present study, fruit extracts of G. asiatica was selected to explore their antioxidant activity. However, few studies speculated the antioxidant property of Grewia asiatica [23]. The present study evaluates and confirms the potential antioxidant activity by different protocols for different free radicals. This study is the cumulative approach to identify potential phytocompounds by GC-MS associated with the antioxidant potential of different extracts.
Collection of Sample
Plant specimens have been collected from the vicinity of Integral University, Lucknow and were identified as Grewia asiatica L. by Birbal Sahni Institute of Palaeobotany, Lucknow, India (Reference sample submitted in the herbarium of BSIP as a specimen).
Preparation of Extracts
The collected and shade dried fruit samples were crushed in pestle mortar to get the fine powder. The extraction was performed with hexane, dichloromethane, methanol and aqueous solvents by the Soxhlet method as described by Nag and his coworkers [24]. The total percent yield of different extracts was measured by following formula:
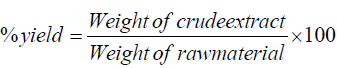
The % yield of different extracts was found as n-hexane: 1.95%, dichloromethane: 3.2%, Methanol: 8.30% and aqueous 4.9%.
Phytochemical Estimation
Determination of total phenolic content (TPC)
Total phenols in fruit samples were determined by the Folin-Ciocalteu method given by [25]. The standard curve was prepared using 12.5 μg/mL to 500 μg/mL solution of Gallic acid in methanol. Total phenol values are expressed in terms of Gallic acid equivalent (μg/mg of dry mass).
Determination of total flavonoid content (TFC)
The quantitative estimation was performed spectrophotometrically by the aluminium chloride method based on the formation of complex flavonoid-aluminium [20]. The calibration curve was prepared with Quercetin standard solutions at various concentrations 12.5 μg/mL to 500 μg/mL in methanol. Total flavonoid values are expressed in terms of Quercetin equivalent (μg/ mg of dry mass).
Antioxidant Assay
DPPH radical scavenging activity
Antioxidant potential was assessed according to previously reported methods of 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical inhibition [26]. The different concentrations of all fruit extracts and ascorbic acid were prepared in methanol. The free radical scavenging activity was calculated in terms of percent inhibition by using following formula:

Where, AControl is the absorption of Control and ASample refer for different test sample.
Further, IC50 values represent the concentration of the extract exhibiting 50% inhibition of DPPH radicals.
ABTS radical scavenging activity
ABTS (2,2'-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) free radical scavenging activity was analyzed by following the standard protocol of Re et al. [26]. The percentage inhibition was measured using following formula:
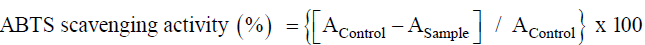
Where, AControl is the absorption of Control and ASample refer for different test sample.
Hydroxyl radical scavenging assay
In-vitro hydroxyl radical scavenging assay was analyzed by standard protocol of [27]. During the analysis different concentrations of G. asiatica fruit extracts were analyzed. The percent inhibition of OH radicals was calculated by following formula and further, IC50 value represents the concentration of the extract exhibiting 50% inhibition of Hydroxyl radicals. Percent Hydroxyl radical scavenging activity was calculated by following formula:
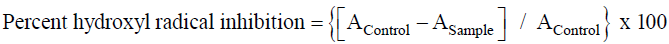
Where, AControl is the absorption of Control and ASample refer for different test sample.
Ferric Reducing Antioxidant Potential (FRAP)
The ability to reduce ferric ions was measured for different solvent extracts of G. asiatica using the method described by Benzie and Strain [28]. Fresh working solutions of FeSO4 were used for calibration. The antioxidant capacity based on the ability to reduce ferric ions of the sample was calculated from the linear calibration curve and expressed as μmol Fe+2 equivalents per μg of the sample.
Superoxide radical scavenging activity
The scavenging assay of superoxide radical was carried out by the standard protocol of Kunchandy and Rao [29]. Different concentrations of test samples were used for the assay and ascorbic acid used as a standard. The percentages of superoxide radical scavenging were calculated by using following formula:
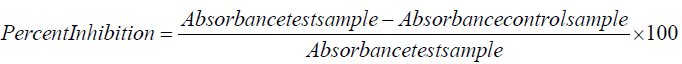
Gas chromatography mass spectrometry analysis
The dried powder of methanol extract was dissolved in the respective solvent and GC-MS analysis of sample was carried out. The sample (1 μL) was injected into a RTX-5 column of GC-MS (model GC-MS-QP-2010 plus, Shimadzu Make). Helium, a carrier gas, was used at a constant column flow of 1.2 mL/min. Temperature programming was maintained from 100°C to 200°C with constant rise of 5°C/min and then held isothermal at 200°C for 6 min. further the temperature was increased by 10°C/min up to 290°C and again held iso-thermal at 290°C for 10 min. The ion source and injector temperatures were 250°C and 270°C, respectively. Mass spectra were taken at fragments from 40 to 950 Dalton and scan at interval of 0.5s at 70 eV. The constituents were finally confirmed by computer matching of the mass spectra of peaks with the Wiley and National Institute Standard and Technology (NIST) libraries mass spectral database.
Statistical analysis
All the data are reported as mean ± SD. The mean values were calculated based on the data taken from at least three independent experiments conducted on separate days. Statistical analysis was done with ANNOVA statistical package. All samples were prepared and analyzed in triplicates.
Estimation of Phenolic and Flavonoid Content
Maximum phenolic (22.9 ± 0.045 μg) and flavonoid (76.69 ± 0.038 μg) was estimated in the MeOH extracts of fruit (Table 1), followed by aqueous, dichloromethane and hexane extracts. The phenolic and flavonoid contents were expressed in equivalent to Gallic acid and Quercetin respectively.
| Extracts | FRAP value µmole (Fe(II)/mg) | TPC (µg/ Gallic Acid/ mg extract) |
TFC (µg/Quericitin/mg extract) |
|---|---|---|---|
| n-hex | 0.020 ± 0.03 | 4.0 ± 0.03 | 4.9 ± 0.065 |
| DCM | 0.04 8± 0.05 | 7.15 ± 0.05 | 9.48 ± 0.04 |
| MeOH | 7.68 ± 0.04 | 22.9 ± 0.062 | 76.69 ± 0.06 |
| Water | 3.20 ± 0.6 | 8.29 ± 0.033 | 31.2 ± 0.038 |
| Ascorbic acid | 12.76 ± 0.54 |
All experiments were performed in triplicates and values are expressed in mean value of ± S.D.
Table 1. Phytochemical estimation with FRAP values.
Antioxidant activity
The DPPH method of antioxidant activity was revealed MeOH extract of fruit for the maximum percent of free radical scavenging activity, i.e. 90.1% (IC50 -62.75 ± 0.06 μg) as compared to aqueous 65.09%, dichloromethane 39.4% and hexane 19.35% respectively (Table 2). Ascorbic acid was used as a standard reference compound which exhibited 96.49% (IC50-5.4 ± 0.05 μg) of free radicals (Table 3).
| Extracts/Standard | Percent Inhibition of free radicals by fruit extracts against various assays at various concentrations (µg/ml) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DPPH | ABTS | H2O2 | Super oxide | |||||||||
| 12.5 | 250 | 500 | 12.5 | 250 | 500 | 12.5 | 250 | 500 | 12.5 | 250 | 500 | |
| n-hexane | 1.2 | 12.73 | 19.35 | 1.02 | 14.53 | 24.08 | 0 | 9.4 | 12.6 | 1.8 | 19.5 | 21.2 |
| Dichloromethane | 3.2 | 19.81 | 39.4 | 3.52 | 24.52 | 31.27 | 5.55 | 51.7 | 54.2 | 3.8 | 25.1 | 31.8 |
| MeOH | 21.42 | 43.91 | 90.10 | 8.81 | 59.76 | 65.98 | 15.92 | 83.9 | 91.1 | 13.2 | 63.2 | 71.5 |
| Aqueous | 2.8 | 27.81 | 65.00 | 6.31 | 29.8 | 52.71 | 7.1 | 53.33 | 78.4 | 5.9 | 52.9 | 57.3 |
| Ascorbic acid | 77.68 | 91.02 | 96.49 | 13.78 | 63.74 | 79.9 | 26.51 | 84.8 | 95.7 | 17.5 | 70.8 | 78.2 |
All experiments were performed in triplicates and values are expressed in mean value of ± S.D.
Table 2. Antioxidant assay of samples.
At a maximum concentration (500 μg/mL) MeOH leaf extract inhibited 65.98% of ABTS free radicals followed by aqueous, hexane and dichloromethane extracts. Whereas standard ascorbic acid inhibited 79.9% ABTS free radicals at the same concentration (Table 2). The IC50 values were also calculated in which MeOH extracts shown 224 μg ± 0.032 μg followed by aqueous extract 431.4 μg ± 0.05 μg, whereas standard ascorbic acid was shown 96 μg ± 0.033 μg IC50 values (Table 3).
| Extracts/Standard | DPPH | ABTS | H2O2 | Super oxide |
|---|---|---|---|---|
| G. asiatican-hex | NA | NA | NA | NA |
| G. asiaticaDCM | NA | NA | 248.7 ± 0.075 | NA |
| G. asiaticaMeOH | 62.75 ± 0.06 | 224 ± 0.032 | 43.8 ± 0.03 | 53.6 ± 0.05 |
| G. asiaticaWater | 79.10 ± 0.09 | 4314 ± 0.05 | 238 ± 0.06 | 246.8 ± 0.08 |
| Ascorbic acid | 5.40 ± 0.05 | 96 ± 0.033 | 32.5 ± 0.04 | 35.3 ± 0.065 |
All experiments were performed in triplicates and values are expressed in mean value of ± S.D.
Table 3. IC50 Values of different antioxidants assays.
Different extracts of fruit have also been examined for H2O2 free radical scavenging method. In this assay MeOH extract identified as a most potent inhibitor for hydroxyl radicals which inhibited maximum percentage 91.1% of H2O2 generated free radicals (IC50-43.80 μg ± 0.03 μg) followed by aqueous (78.4%, IC50-238 μg ± 0.06 μg), dichloromethane (54.2%, IC50-248.70 μg ± 0.075 μg) and n-hexane (12.6%) extract (Tables 2 and 3).
The study illustrated that MeOH extract has significantly higher FRAP values (7.68 μmol ± 0.04 μmol Fe(II)/mg) as compared to other extracts, however standard ascorbic acid reduced maximum ferric ions (12.76 μmol ± 0.54 μmol Fe(II)/mg) (Table 1).
Our results found that maximum superoxide radical scavenging capacity was shown by MeOH extract 71.5% (IC50- 53.6 μg ± 0.05 μg) followed by aqueous 57.3% (IC50-246.8 μg ± 0.08 μg), dichloromethane 31.8% and n-hexane 21.2% extract at the concentration of 500 μg/mL (Table 2). The linear correlation between the total phenols, total flavonoids and the FRAP value was shown in Figure 1 and coefficient of determination was 0.827 and 0.956 (respectively for TPC and TFC) between the TPC, TFC and FRAP. This activity indicates that phenolic and flavonoids contents in the extracts are responsible for the scavenge free radicals.
Gas chromatography and mass spectrometry (GC-MS) analysis
The spectral results of GC-MS analysis were compared with NIST/Wiley library. Thirteen compounds have been identified in the MeOH fruit extract of G. asiatica named as 2-furancarboxaldehyde, 5-(hydroxymethyl (62.22%), 2,3-dihydro-3,5-dihydroxy-6- methyl-4h-pyran (11.05%), 1,3,5-triazine-2,4,6-triamine (5.10%), 3-methyl-2,5-furandione (4.13%), Pentadecanoic acid (2.93%), 2,4-dihydroxy-2,5-dimethyl-3(2h)-furan-3-one (2.18%), 4-oxopentanoic acid (1.58%), cis-dimethyl morpholine (1.47%) Hydrazine carboxamide, 2-(2-ethylcyclohexylidene)-(1.43%) Oxy bis-(methyl) (0.79%) furancarboxaldehyde, 5,5'-(0.79%) Cis-vaccenic acid (0.40%) Cyclo-hexane carboxylic acid (0.56%) (Figure 2 and Table 4). Mostly identify compound are known as phenol, flavonoid and aldehydes. The chemical formula of this compound was identified on the basis of NIST and Wiley library.
| S. No | R. Time | % Area | Name | Activity |
|---|---|---|---|---|
| 1 | 13.163 | 62.22 | 2-furancarboxaldehyde, 5-(hydroxymethyl) | Antioxidant, Anti-microbial |
| 2 | 10.150 | 11.05 | 2,3-dihydro-3,5-dihydroxy-6-methyl-4h-pyran | Anti-diabetic, Anti-oxidant |
| 3 | 8.413 | 5.10 | 1,3,5-triazine-2,4,6-triamine | Anticancer, Anti-microbial |
| 4 | 4.672 | 4.13 | 3-methyl-2,5-furandione | Antioxidant |
| 5 | 29.610 | 2.93 | Pentadecanoic acid | Anti-microbial, Anti-oxidant, Anti-cancer |
| 6 | 5.404 | 2.18 | 2,4-dihydroxy-2,5-dimethyl-3(2h)-furan-3-one | Anti-microbial |
| 7 | 7.206 | 1.58 | 4-oxopentanoic acid | Anti-microbial, Anti-cancer |
| 8 | 15.217 | 1.47 | cis-dimethyl morpholine | Anti-fungal |
| 9 | 24.286 | 1.43 | Hydrazine carboxamide, 2-(2-ethylcyclohexylidene)- | Anti-microbial |
| 10 | 31.596 | 0.79 | Oxy bis-(methyl) | Insecticide |
| 11 | 30.073 | 0.79 | 2-furancarboxaldehyde, 5,5'- | Anti-microbial, preservative |
| 12 | 34.415 | 0.56 | Cyclo-hexane carboxylic acid | Anti-microbial Preservative |
| 13 | 34.201 | 0.40 | Cis-vaccenic acid | Anti-inflammatory |
Table 4. GC-MS identified compound of methanolic fruit extract.
In present study diversity of phytocompounds were extracted with hexane, dichloromethane, MeOH and aqueous solvents from fruits and has been characterized as a total phenolic and flavonoid content. Zia-ul-Haq and coworkers [22] reported the maximum isolation of these phytocompounds in methanol solvent from G. asiatica. Other phytochemical studies related to bioactive compound isolation have reported maximum phenol and flavonoid in methanol solvent [30]. When the total phenolic content of methanol fruit extract was compared with other solvent extracts, aqueous extract was shown second higher phenolic content which was followed by ethanol, chloroform, dichloromethane and hexane. This confirmed the isolation of phytocompounds on the polarity based extraction. However, the polarity of the respective solvent plays a key role to extract more specific bioactive compounds (phenols and flavonoids) for potential antioxidant activity [31,32].
Plant phenols are a significant group of compounds which act as a free radical scavenging. Polyphenolic compounds have an aromatic benzene ring substituted with hydroxyl groups, including their functional derivatives. Which are able to neutralize free radicals and can chelate various metal ions that could initiate the generation of ROS which activates lipid peroxidation. Among all types of phytochemical polyphenols, flavonoids are important because they protect the human beings to fight against disorders. The molecular structure of flavonoids helps in the antioxidant activities. These phytocompounds are abundantly found in plants as their glycoside [33]. The most abundant flavonoid which has effective antioxidant property is quercetin, as it has all correlative structural orientations for scavenging the free radicals [34]. Therefore, it can be said that phenols and flavonoid content may work together with other phytochemicals present in G. asiatica and make it medicinally important.
In the present study, the antioxidant potential of G. asiatica was measured using different assays, namely FRAP, DPPH, ABTS, Hydroxyl radical and superoxide antioxidant activity. A single antioxidant assay would not be able to evaluate correct results of antioxidant properties of plant extract. Therefore, it is necessary to assay more than one type of antioxidant capacity potential [35]. DPPH is nitrogen-centered free radical having an odd electron which gives a strong absorption at 517 nm, its color changes from purple to yellow when DPPH odd electron paired off in the presence of radical scavenger to form the reduced DPPH-H. ABTS assay depends on the antioxidant compound`s ability to scavenge ABTS radical. By this assay, we can measure the antioxidant capacity of lipophilic and hydrophilic compounds in the same sample.
The hydroxyl radical is also one of the important reactive oxygen species in the biological system which interacts with fatty acid complexes of the cell membrane and damaged the cell [36]. Hydrogen peroxide naturally present in air, water, plants, foods and human body at low concentrations [37]. H2O2 rapidly generated OH radicals, which cause lipid per oxidation as well as DNA damage [38].
Ferric reducing antioxidant power (FRAP) assay depends on the reduction of the ferric ion into a ferrous ion [28]. A linear correlation study also suggested phenolic and flavonoid contents are responsible for the potent antioxidant activity.
The superoxide radical assay is known as an important source of reactive oxygen species in the biological system [39]. Superoxide anion generated hydroxyl radicals which are responsible for oxidative stress [40]. Our results suggest that phenolic and flavonoids content present in methanol extract may be the major contents of the antioxidant activity as the IC50 values of radical scavenging activity of all extracts of G. asiatica and phenolics or flavonoids contents exhibited a significant correlation. Above all activities were found significant in the methanolic fruit extract of G. asiatica. Furthermore, GC-MS analysis was carried out to identify the compounds in methanolic extract which revealed various compounds. There were thirteen compounds have been identified in which furan carboxaldehyde, 5-(hydroxymethyl) having maximum (62.22%) percent area followed by 2,3-dihydro-3,5-dihydroxy- 6-methyl-4h-pyra (11.05%) and other compounds. During the analysis methanol extract was shown maximum antioxidant activity which may be due to the presence of these compounds and may be a synergistic effect. The individual compound of GC-MS analysis has been reported in previous studies for antioxidant, anti-inflammatory, antimicrobial and preservative activity [41,42]. Based on various free radical scavenging activities results are significant for methanolic extract of G. asiatica which may be due to the presence of phenol and flavonoids in the fruit extract [43].
The authors would like to thank honorable vice-chancellor, Prof. S. W. Akhtar, for providing laboratory facilities to do research work. We also want to acknowledge Dr. Ajay Kumar (AIRF, JNU) for GC-MS analysis.