ISSN: 2320-0189
ISSN: 2320-0189
1Department of Horticultural Plant Production, Islamic Azad University, Iran
2Department of Horticulture, Islamic Azad university, Iran
Received date: 17/09/2019; Accepted date: 09/03/2020; Published date: 16/03/2020
Visit for more related articles at Research & Reviews: Journal of Botanical Sciences
Objective: Cilantro (Coriandrum sativum L.) is an annual herbaceous plant of the umbrella family which widely used in the food, cosmetic and pharmaceutical industries. The presence of plumbum (Pb) in the environment, a major environmental pollutant, has devastating and irreversible effects on the ecosystem. This study aimed to examine the effect of different levels of plumbum and Trichoderma fungus "Trichoderma harzianum" on the growth and physiological properties of Cilantro. Methods: In this context, an experiment was conducted as a factorial in the form of completely randomized design with three replicates to examine the effects of plumbum in three levels and Trichoderma fungus in two levels on coriander plant and phytochemical properties and plumbum were measured. Results: The results showed that the highest chlorophyll a, b and total chlorophyll (1.644, 0.892 and 2.345 mg/g of fresh weight) was observed in the interactions between plumbum (0 mmol/L) and 20% of Trichoderma treatment. Also, the highest amount of anthocyanins (0.049 mmol/g of fresh weight) and essential oil percent (0.525%) was observed in the interaction between plumbum (2 mmol/L) and 20% of Trichoderma treatment. The highest amount of linalool (43.869%) was observed in the interaction between plumbum (0 mmol/L) and 20% of Trichoderma treatment. Also, percentage of free radicals (49.871) was observed in the interactions between plumbum (0 mmol/L) and 20% of Trichoderma treatment. Conclusion: The results of this study showed that treatments with interaction plumbum and Trichoderma fungus can be a useful and better promising.
Cilantro, Antioxidant, Essential Oils, Trichoderma, Plumbum, Chlorophyll, Linalool
Cilantro, also known as “Coriandrum sativum L.” is an annual herbaceous plant of the umbrella family which has its leaves and fruits containing essential oil which is used increasingly in the food, cosmetic, beverage, chocolate and pharmaceutical industries [1]. It is native to the Mediterranean and Middle Eastern regions and has been known in Asian countries for thousands of years. It is indigenously distributed in Italy, but widely cultivated in the Netherlands, Central and Eastern Europe (Russia, Hungary and Holland), Mediterranean (Morocco, Malta and Egypt), North Africa, China, India and Bangladesh. Linalool, present in Cilantro fruits compositions, is another form of essential oil with new potential that is greater when compared to other plants [2]. In addition to pollution caused by oil and industrial fuel, the pollution of water and soil with heavy metals is one of the major environmental problems in human societies which cause decline in the performance and quality of product and sustainable agriculture, and also endangers human health [3]. Among heavy metals, cadmium and plumbum possess significant toxicity and biological half-life of approximately 20 years due to their high mobility in the soil and ease of absorbance by plants [4]. The use of live root system of the plant facilitates the transfer of heavy metals from the soil to the plant, and during harvest of organs the retaining of these metals is practically possible in contaminated areas [5]. Given the mentioned cases, the management of fertilizers is an important factor in the success of plants cultivation. Also, identifying the fertilizers that are consistent with nature and the plants, have beneficial effects on the qualitative and quantitative indicators of plants, and it seems that the organic production of these plants is the perfect solution for the production of healthy plants. Organic materials such as vermicompost and Trichoderma fungus, improve the physical properties of the soil, provide nutrients for plants and also reduce the consumption of mineral fertilizers. High consumption and accumulation of organic nitrogen creates nitrogen organic bonds in the soil and reduce the nitrogen leaching after mineralization. The extensive and unconventional use of chemical fertilizers and pesticides results in soil salinization and destruction of aggregation [6]. Perhaps, there is a relationship between production and stimulation of secondary metabolites and Trichoderma fungus in plants under the influence of plumbum, in the other hand, increase of Trichoderma fungus on several plants was investigated [7]. Totally, there is little evidence on the effect of Trichoderma fungus on some of the morphological and biochemical properties, essential oil production and the secondary metabolites and in this field, the application of Trichoderma fungus under the influence of heavy metals such as plumbum can be noted as a beneficial solution in the novel agricultural activities on medical plants. Given the importance of Cilantro as a medical plant, examining the factors affecting its qualitative and quantitative performance is important for proper monitoring of the environmental effects through some methods; therefore, the potential of the affected plant can be indicated. This study aimed to examine the effect of different levels of plumbum and Trichoderma fungus on the growth and physiological properties of Cilantro. Some new potentials of this plant has been explained, including the application of Cilantro in cleaning of the contaminated environment due to its hyper accumulator property of collecting heavy metals (including arsenic, aluminum, plumbum, cadmium, zinc and nickel). Also, the effect of plumbum and Trichoderma fungus in different concentrations is examined on some of the photochemical properties of Cilantro.
Experimental Procedures
The seeds of Cilantro were prepared from the store. Before culture, a soil sample that used as a field soil in all pots was sent to the soil laboratory for physical and chemical analysis. Fifteen (15) seeds were harvested and irrigated immediately at a depth of 3 cm in the pots with opening diameter of 12 cm and height of 15 cm. After germination, the week seedlings were removed in stage 4 and finally the number of plants in each plot was reduced to five. This study was conducted as a factorial in the form of completely randomized design to examine the effects of plumbum in three levels of "zero, 2 and 4 mmol/L" and trichoderma fungus in two levels of "0% and 20%" on Coriander plant in the pot. Treatments were applied at approximately 5 leaf stage and once every 6 days for 1 month.
Measurement of Biochemical Contents of Cilantro Plant
Measurement of carotenoids and chlorophylls content
Chlorophylls content was extracted by a mixture of acetone and water at a ratio of 20%-80% (v/v). 2 g of Cilantro’s homogenized tissue with 25 ml acetone solution 80% was meshing using laboratory blender for 2 min. Extract was centrifuged at 2700 rpm for 10 min and then, took 3 ml of supernatant extract. Chlorophyll a has approximate absorbance maxima of 430 nm and 662 nm, while chlorophyll b has approximate maxima of 453 nm and 642 nm, so absorption was measured at 663, 645 and 470 nm using S 2000 uv/vis spectrophotometer concentration of chlorophyll (a, b) and total carotenoid was reported as mg /g fresh weight. Chlorophyll a, b and carotenoids measured using the formula by Arnon [8].
Carotenoid mg in per gram of fresh tissue = [7/6 (A480)-1.49 (A510)] × V/1000W
Chollorophyl a mg in per gram of leaf tissue] = [12.7 (A663)-2.69 (A645)] × V/1000W
Chollorophyl b mg in per gram of leaf tissue = [22.9 (A645)-4.68 (A663)] × V/1000W
Total hollorophyl a mg in per gram of leaf tissue = [20.2 (A645)+8.02 (A663)] × V/1000W.
Measurement of the amount of antioxidant activity
DPPH assay: The DPPH test was carried out using method described by Cuendet and Kirby, 0.1 ml methanol extract was prepared and mixed with 1 ml of a 0.1 mm of DPPH. After 30 min incubation, the absorbance of the samples was read at 517 nm using 2000 uv/vis spectrophotometer. 80% methanol was used as positive controls and total antioxidant was calculated using the final formula:
Percent of inhibition DPPH free radicals = 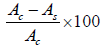
Ac: The absorbance of the control sample
As: The absorbance of each sample [9,10].
Measuring the amount of plumbum
0.2 g of dried plant samples were placed into the test tube and mixed with 2 ml of concentrated nitric acid. After 24 h, 2 ml of hydrogen peroxide was added to each sample in order to complete the tissue digestion and the mixture was heated in a water bath at a temperature of 70ºC for 20 min to obtain a final clear and colorless mixture. At the end, the solution was filtered using a filter paper and was made up to a volume of 25 ml with distilled water. Measurement of the concentration of plumbum in the samples was performed using atomic absorption spectrometry [11].
Measurement of anthocyanin
Measurement of anthocyanin was done using the Wagner method. All samples were weighed and 0.1 g of each samples poured in 10 ml methanol/HCl solution [methanol: HCl 99:1 v/v]. The tissues were crushed and kept at dark place under ambient temperature for 24 h. After that, the extracts were centrifuged at 4,000 g for 10 min at room temperature. The absorption rate of the supernatant was read by spectrophotometer at 520 nm. To calculate the amount of anthocyanins, the extinction coefficient 33,000 mMcm-1 was used and anthocyanin content was expressed as μ mol g fw [12].
Determination of flavonoid content
The aluminum chloride colorimetric method by Woisky and Salatino was modified and used for determination of flavonoid content. The calibration curve was drawn using quercetin. 10 mg of quercetin was dissolved in 80% ethanol and then diluted to 50, 100 and 250 μg/mL. 4 samples of the standard solutions were prepared. It was made by mixing 0.5 ml of the diluted standard solutions with 0.1 mL of 10% aluminum chloride, 1.5 mL of 95%ethanol, 0.1 mL of 1 m potassium acetate and 2.8 mL of distilled water. Aluminum chloride was replaced by distilled water. Determination of flavonoid content was done by reaction of aluminum chloride and of ethanol extracts. After 30 min incubation at ambient temperature, the absorbance of the reaction mixture was measured at 415 nm using as 2000 uv/vis spectrophotometer [13].
Determination of total phenolic content
Total phenol in leaf extract was determined by Folin Ciocalteu’s method. 0.5 mL extract mixed with 7 mL sterile distilled water in a test tube and then 0.5 ml of Folin Reagent added. After 3 min, 1 ml of saturated sodium carbonate solution was added to the sample and the solution volume was increased to 10 ml by distilled water. After 1 h, the absorbance was read at 765 nm using S2000 uv/vis spectrophotometer. The total phenolic content of the extract was measured using a standard curve based on mg of Gallic acid per gram of extract (The control tubes included distilled water and reagent) [14].
Extraction and determination of essential oil
To prepare the essential oil, 100 g of dried aerial parts of Cilantro was powdered and added to hydro-distillation for 3 h using a Clevenger apparatus. The essential oil obtained from three replicates per treatment was collected in separate micro tubes. After dehumidification with sodium sulfate, the essential oil was placed in the refrigerator at temperatures range of 4 to 5 ºC until the measurement time of Linalool.
Measurement of linalool
6 μl of the essential oil from each sample was diluted with acetonitrile to 10 cc, all samples were filtered and injected into the HPLC apparatus three times. The HPLC system used throughout this study was a Lacrom pumps model L-7100 and Lacrom detectors model L-7100 with an auto-injector and a waters reversed-phase C18 analytical column (4.6 mm × 250 mm, 4 μm).
Linalool was obtained from Sigma. HPLC-grade methanol and acetonitrile were purchased from Merck (Germany). Deionized water was used for all experiments. A stock solution of linalool (1,000 μg/mL) in methanol was prepared and kept at 4 ºC. Serial dilutions of stock solution with methanol were used to making the calibration standard solutions (100–250 μg/mL). The mobile phase was acetonitrile-water (50:50 v/v) with a flow-rate of 1 mL/min. The mobile phase was degassed with ultrasonic and the absorbance was measured at 210 nm.
Statistical Calculation
The experimental design was completely randomized factorial with three replications. Data analysis and average comparison was done using SAS software while Excel software was used to draw the diagrams. Means were compared using the Least Significant Difference Test (LSD) at 5% probability level.
The Results of Measured Indicators
Evaluation of different levels of plumbum and Trichoderma Fungus on morphological traits in Cilantro
According to the Table of variance analysis, the treatment of different level of plumbum, Trichoderma fungus and their interaction together on plant height, fresh weight of aerial organs, dry weight of aerial organs and the number of leaves had significant effect in a probability level of 5% Table 1.
| S.O.V | df | Plant height (cm) | Fresh weight of aerial organs | Dry weight of aerial organs | The number of leaf |
|---|---|---|---|---|---|
| Plumbum | 2 | 13/443* | 0/498* | 0/432* | 5/254* |
| Trichoderma | 1 | 6/598* | 0/534* | 0/232* | 1/155* |
| Plumbum* trichoderma | 2 | 14/764* | 0/212* | 0/113* | 0/985* |
| Error | 12 | 45/67 | 34/56 | 6/987 | 2/874 |
| CV (%) | - | 4/859 | 7/946 | 3/675 | 7/878 |
** and * significant at P = 0/01 and P= 0/05, %, n.s lack of significant level
Table 1: Analysis of variance (ANOVA) of different levels of Plumbum and Trichoderma Fungus on morphological traits in Cilantro.
Plant height in different levels of Plumbum treatments
The maximum height of plant was observed in control treatment (16/453 cm) and the lowest plant height was related to the "Lead stress" treatment (15/024 cm).
Plant height in different levels of Trichoderma Fungus treatments
The maximum height of plant was observed in 20% level of Trichoderma treatment (14/493 cm) and the lowest plant heights were belonged to the control treatment (10/047 cm).
Plant height in Interaction plumbum and Trichoderma treatments
The results show that the maximum height of plant was related to the interaction between control treatment and 20% Trichoderma fungi (13/135 cm).
Fresh and dry weight of aerial organs in different levels of plumbum treatments
According to the research results, the maximum amount of fresh weight of aerial organs observed in control treatment (2/963 g) and the minimum of them (2/584 g) related to plumbum (4 mmol/L) treatment. The maximum of dry weight of aerial organs was belonged to plumbum (0 mmol/L) treatment (2/394 g) and the minimum of the amount of dry weight of aerial organs was related to plumbum (4 mmol/L) treatment (2/024 g).
Fresh and dry weight of aerial organs in different levels of Trichoderma fungus treatments
The maximum amount of fresh and dry weight of aerial organs observed in 20% of Trichoderma treatment (1/890 g) and (1/553 g). Also, the minimum of them (1/788 g) and (1/239 g) related to control treatment.
Fresh and dry weight of aerial organs in Interaction plumbum and Trichoderma treatments
In similar results it was seen that the maximum amount of fresh and dry weight of aerial organs observed in interaction between plumbum (0 mmol/L) and20% of Trichoderma treatment (2/137 g) and (1/452 g) and the minimum of them (0/770 g) and (0/658 g) related to interaction between control treatment and plumbum (0 mmol/L) treatment.
Number of leaf in different levels of plumbum treatments
The maximum number of leaves was observed in control treatment (6/97 numbers) and the minimum number of leaves was related to the plumbum (4 mmol/L) treatment (4/02 numbers).
Number of leaf in different levels of Trichoderma Fungus treatments
The maximum number of leaves was observed in 20% of Trichoderma treatment (5/562 numbers) and the minimum of leaves was observed in the control treatment (4/846 numbers).
Number of leaf in Interaction plumbum and Trichoderma treatments
Based on the results, the maximum number of leaves was related to the interaction between plumbum (0 mmol/L) treatment and 20% Trichoderma fungi (6/563numbers) and minimum of them (4/034 numbers) belong of interaction of plumbum (4 mmol/L) treatment and control treatments.
Examining the Different Level of Plumbum and Trichoderma Fungus on Chlorophyll (Total, A and B), Carotenoids, Plumbum, Anthocyanin, Essential Oil and Linalool Amount
Table of variance analysis showed that the treatment of different level of plumbum and Trichoderma fungus, together with their interaction on Chlorophyll a, Chlorophyll b, total Chlorophyll, carotenoid, plumbum, anthocyanin, essential oil and linalool had significant effect in a probability level of 5% Table 2.
| Resource of changes | Linalool (percent) | Essential oil (percent) | Plumbum (mg/kg) | Anthocyanin (mmol/g) | Carotenoid (mg/g) | Total Chlorophyll (mg/g) | Chlorophyll b (mg/g) | Chlorophyll a (mg/g) | df |
|---|---|---|---|---|---|---|---|---|---|
| Plumbum | 42.762* | 0.661* | 1.698* | 0.009* | 0.800* | 4.089* | 0.809* | 1.09* | 2 |
| Trichoderma | 34.772* | 0.764* | 0.453* | 0.005* | 0.504* | 5.003* | 0.604* | 2.01* | 1 |
| Plumbum* | 29.658* | 0.351* | 0.862* | 0.076* | 0.782* | 6.002* | 0.322* | 1.01* | 2 |
| Trichoderma | 57.884 | 56.876* | 43.421* | 67.333 | 0.636 | 8.055 | 0.098 | 3.98 | 12 |
| Coefficient changes (CV %) | 9.384 | 3.985 | 5.765 | 2.877 | 1.369 | 8.636 | 4.689 | 5.559 | - |
** Significant different at 1% level, * significant level at 5%, n.s lack of significant level
Table 2: Analysis of Variance (ANOVA) of chlorophyll (total, a and b), carotenoids, plumbum, anthocyanin, essential oil and linalool under different treatment of plumbum and Trichoderma.
Anthocyanin
According to the average comparisons, the highest amount of anthocyanin (0.049 mmol per g of fresh weight) was observed in the interaction of plumbum (2 mmol/L) and 20% of trichoderma treatment.
Chlorophyll
The highest amount of chlorophyll b (0.892 mg per fresh weight gram) was observed in the interaction of plumbum (0 mmol/L) and 20% of trichoderma treatment. Also, the highest amount of total chlorophyll (2.345 mg per fresh weight gram) was observed in the interaction of plumbum (0 mmol/L) and 20% of trichoderma treatment.
The amount of plumbum
According to the average comparisons, the maximum amount of plumbum (5.654 mg per kg of dried weight) was observed in the interaction of plumbum (4 mmol/L) and 0% trichoderma treatment. The lowest amount of plumbum (0.001 mg per kg of dried weight) was observed in the interaction of plumbum (4 mmol/L) and 20% of trichoderma treatment.
Essential oil and linalool
According to the average comparisons, the highest amount of essential oil percent (0.525%) was observed in the interaction of plumbum (2 mmol/L) and 20% of Trichoderma treatment. Also, the lowest amount of essential oil percent (0.213 %) was observed in the interaction of plumbum (4 mmol/L) and 0% of Trichoderma treatment and also, the highest amount of linalool (43.869 %) was observed in the interaction of plumbum (0 mmol/L) and 20% of trichoderma treatment. Also, the lowest amount of linalool (24.245%) was observed in the interaction of plumbum (4 mmol/L) and 0% of Trichoderma treatment.
The Effect of plumbum and Trichoderma fungus on antioxidant Activity
According to table of analysis of variance, the examined treatments had significant effect on the measured variables; so that, plumbum and Trichoderma fungus had effect on the free radicals and IC 500 in a probability level of 5%. Also, plumbum and Trichoderma fungus had significant effect on free radicals and IC 500 in a 1% probability level.
The highest percentage of free radicals (49.871 %) was observed in the interaction of plumbum (0 mmol/L) and 20% of Trichoderma treatment. And also, the lowest percentage of them (1.473 %) was observed in the interaction of plumbum (4 mmol/L) and 0% of Trichoderma treatment Table 3.
| Resource of changes | IC50 | Percent of free radicals | df |
|---|---|---|---|
| Plumbum | 111.91* | 343.67* | 2 |
| Trichoderma | 74.85* | 298.12* | 1 |
| Plumbum * Trichoderma | 43.56** | 178.11* | 2 |
| Error | 43.565 | 89.455 | 12 |
| Changes coefficient (%) | 9.567 | 5.997 | - |
Table 3: Variance Analysis of Antioxidant Activity Under Plumbum and Trichoderma Treatment.
Measurement of linalool
Figure 1 showed that, the linalool standard curve had R=0.9984 and chromatogram showed an increase of peak area of linalool precisely at 10 μL of pure linalool Figure 1.
Plumbum is one of the major pollutants in the environment. The accumulation of this element in the environment, which has a long-lasting life, endangers human, animals and plants health [15]. Availability of plumbum soluble forms leads to uptake this element by plant's roots. Plumbum toxicity leads to inhibition of enzyme activities, change in hormonal status and alteration in membrane permeability, decrease in leaf area, reduce intercellular spaces and intensity of transpiration are due to heavy metals in the plant which reduce water absorption and can affect photosynthetic system and at high concentrations , causes cell death [16].
Stress causes plants to wilt which activate the plant's defense mechanism. Secondary metabolites production is one of the environmental stresses tolerance mechanisms in plants. Some species of fungi such as Trichoderma spp. have been reported to improve plant resistance to environmental stress and induction of plant defense responses against stressful condition such as high level of lead in soil. Many studies have been conducted on the influence of Trichoderma fungi on improvement of Plant performance [17]. This fungus coexists with the root of the plant and able to colonize plant roots and also Induced Systemic Resistance (ISR) of plants against pathogens through the production of various chemicals compounds [18]. This study aimed to evaluate the effect of “T. harzianum” on antioxidant activity and Phytochemical Parameters of Cilantro in the presence of various Plumbum levels. A similar study was done on the effect of “T. harzianum” on Cauliflower (Brassica oleracea convar. botrytis), that the results showed that the interactive effects of fungi and plumbum in different concentrations can be effective on increasing the amount of chlorophyll, carotenoids and increasing antioxidant active it [19]. Also in another study in 2018 reported that the toxicity by plumbum leaded to decreasing the root system growth and the increased antioxidant activity of the treatments suggested that the maximum physiological activity and interactions between Trichoderma fungus and the plant occurred in the roots and also all of antioxidant compounds (e.g., phenolic acids, flavonoids, catalase and superoxide dismutase) in treatments with Trichoderma were in high levels which confirms the results of the current research [7]. Some concentration of Plumbum was absorbed in the soil by plant while others remained in the root and moved toward the bottom. Plumbum has negative effect on the plant photosynthesis, which is consistent with the result of the study [20]. This effect is generally due to the increase in the production of abscisic acid by Plumbum which results in the reduction of stomatal conductance and finally reduces photosynthesis [21]. Plumbum affects the photosynthesis system in different ways, for example changing in composition of photosynthesis pigments, reduction in the total chlorophyll, reduction in the ratio of chlorophyll a/b are all consequences of Plumbum on the photosynthesis system of a plant [22]. The toxic effect of Plumbum on plant growth includes prevention of photosynthesis, prevention of absorbing nutrients and imbalance of water relations which results in reduction in vegetative and reproductive growth [23,24]. It was stated that the presence of plumbum in (Ceratophyllum demersum) plant affects the chloroplast [25]. It is clear that, fungus in the root cells neutralizes the radicals. The difference in different levels of secondary metabolites with equal efficiency is acceptable and depends on the plant preference and different interaction in the route of metabolic synthesis [26]. It is normal that plants under stress have maximum antioxidant activity. By increasing the stress, signals of stress are activated and this results in increasing the antioxidant capacity. On other hand, these changes are increased by increasing the activity of the main enzymes involved in antioxidants activities, such as superoxide dismutase (SOD), catalase (CAT) and ascorbate peroxidase (APX) to reduce the effect of stress [27]. Michalak A, reported a high rate of induction of accumulation of phenolic compositions and peroxidase activities in the treated plans. Flavonoids have both hydroxyl and carboxyl groups which have the ability to bond the heavy metals specifically, and the roots of most plants exposed to these heavy metals have high amount of flavonoid production [28]. Bahmanyar examined the effect of wastewater consumption on some heavy metals of soil and plants on crops irrigation and discovered that the amount of cadmium, nickel, chrome and the plumbum accumulated in the root of rice was due to the low transfer of plumbum to shoot, and the transfer in seed was more than shoot and seed in rice [29]. Increasing antioxidant activity in the plant samples indicated the highest physiological activity and fungus interaction in the root; therefore, root is stronger in terms of antioxidant compositions. It is worth noting that in the control samples, the antioxidant ability of plant was reduced by increasing the plumbum. As expected, all antiradicals’ compositions (phenyl, flavonoid and antioxidant) in the treated plants by fungus were maximum and the ability to absorb free radicals was in a higher level, while non-symbiotic plants had lower antioxidant ability in all levels of stress. The experiment of Waller et al. showed that fungus is not a factor to discharge antioxidant enzyme [30]. Jia et al. reported an increase in the antioxidant compositions during colonization of symbiosis had effect on the ROS detoxification and this is a factor for the symbiosis between Entophytes fungus and plant [31]. Shultz et al. conducted a wide experiment on 6500 Entophytes fungus (P. India) and found a brief statistic of secondary compositions which was obtained by a natural living source [32]. These researchers reported that the secondary metabolites are often obtained by different biosynthesis routes such as polysaccharide, isopernoids and amino acid derivations. About 51% of extracted compositions of entophytes activities are still unidentified even in the mentioned years. In this study, it was stated that the defense system of plant, such as polyphenol compositions is reduced during the attack of pathogens or severe environmental stress [33]. While the root caused increase in the plant secondary metabolites as much as control samples in the experiment in symbiosis with endophytic [34]. Sun et al. reported increase of root and shoot growth and production of root hairs by insemination of Chinese cabbage Piriformospora, and induction of drought stress at different levels using poly ethylene glycol [35]. In addition, they reported that peroxidase antioxidant enzymes, catalase and demostasis superoxide were increased within 24 h and the fungus reduced the drought stress and increased the amount of thylakoid proteins and photosynthesis efficiency. Chen et al. in an experiment on the effect of “Funneliformis mossae” on cucumber seedlings under temperature stress of (10 to 15 ºC) showed increase in secondary metabolites, including total phenyl, total flavonoids and antioxidant activity and this is consistent with the results of the present study [36].
The results of this study showed that the effect of the different level of Plumbum and Trichoderma fungus on some of the physiological properties that the treatment with interaction between Plumbum and Trichoderma resulted in increasing the level of chlorophyll, carotenoids, anthocyanin and amount of essential oil, especially linalool and also reducing the percentage of free radicals by increasing antioxidant activity. So, in conclusion, it can be said that treatments with interaction Plumbum and Trichoderma fungus can be a useful and better promising.
The authors thank the Islamic Azad University for financial support.