ISSN ONLINE(2278-8875) PRINT (2320-3765)
ISSN ONLINE(2278-8875) PRINT (2320-3765)
Ola Al-Qasem1 and Jafar Jallad2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Advanced Research in Electrical, Electronics and Instrumentation Engineering
Storage batteries are indispensable in all standalone solar electric systems (PV power systems). Their efficiency and life time affects significantly the overall PV system performance and economics. Batteries specified especially for use in PV systems have to be distinguished with standing of a very deep discharge rate and high cycling stability. A lead acid battery has been exposed to experimental tests to determine its characteristic parameters by charging and discharging processes. The internal resistance of the battery is a reliable key for determination of its state of charge, its value increases with increasing of the stored energy. At the same time the specific gravity of the electrolyte decreases linearly with the degradation of ampere hour capacity. The experiments have shown that the battery internal temperature doesn’t change significantly from the ambient temperature during charge and discharge processes. The experimental tests have proved that a regular lead acid battery cell will be not more rechargeable if it is fully discharged. This issue requires using always a controllable battery charger within the PV power systems to protect the storage batteries against deep discharge and extremely over charge. Such equipment will extend the life time of the battery and consequently improve the economic feasibility and reliability of the PV power systems. In addition, the tests have shown that the watt hour efficiency of a battery is considerably less than the ampere hour efficiency, which advices to depend more on the watt hour efficiency when designing storage battery systems to secure higher reliability. Moreover, depending on an earlier developed algorithm for determination of the ampere hour capacity of a battery cell, a new similar algorithm based on specific gravity and cell voltage have been developed which enables also the determination of the ampere hour capacity from the implemented tests on the new battery. This algorithm enables the correct settings of the limits of charge - discharge hysteresis of the battery charger in order to avoid extremely deep discharge and over charge of the battery.
Keywords |
| Electrical energy storage, photovoltaic power systems, storage batteries. |
INTRODUCTION |
| Some of the issues and aspects associated with the use of lead-acid batteries for energy storage in small PV systems were represented. New and emerging energy storage technologies such as the vanadium redox battery and high-speed flywheel are considered as possible alternative energy storage systems in PV applications. Lead-acid battery is the technology of choice for most PV applications. However, there are performance limitations which result in excessive replacement costs, work-place Occupational Health and Safety (OS& H) issues and operational maintenance overheads for many end-users [1]. The most important characteristics of lead acid batteries necessary for evaluation of their performance were discussed. Moreover, an experimental procedure is illustrated for developing a mathematical algorithm for determining the ampere hour capacity of batteries operating in PV systems. Battery voltage in function of electrolyte temperature, depth of discharge and specific gravity as well as the battery capacity in function of discharge current, have to be given special consideration when evaluating or designing storage batteries for PV power systems [1]. In stand-alone photovoltaic (PV) systems, charge controllers prevent excessive battery overcharge by interrupting or limiting the current flow from the PV array to the battery when the battery becomes fully charged. Charge regulation is most often accomplished by limiting the battery voltage to a predetermined value or cut-off voltage, higher than the gassing voltage. These regulation voltages are dependent on the temperature and battery charge current [2]. The storage of energy in batteries is a cause of the failure and loss of reliability in PV systems. The two general lead acid battery models and their agreement with experimental data were reviewed. In order to validate these models, the behavior of different battery cycling currents has been simulated [3]. |
| Storage batteries occupy a very wide range of applications but we can’t list every single application that uses batteries. What follows is a brief survey of some of the applications, especially those that require some specialized batteries: |
| • Portable consumer devices (mobile power for an unplugged society, power tools) such as laptop computers, electronic games, battery-operated toys, and flashlights. |
| • Medical devices: life-sustaining and life-enhancing medical devices, including pacemakers, defibrillators, hearing aids, pain management devices, and drug pumps. |
| • Electric vehicles, including hybrids vehicles. Rechargeable batteries are used for automobile starters, portable consumer devices, light vehicles (such as motorized wheelchairs, golf carts, electric bicycles, and electric forklifts), tools and uninterruptible power supplies. |
| • Large-scale energy storage. |
| • Space Satellites require battery power when they enter the Earth shadow and their solar panels do not function. |
| • Military batteries: Like space batteries, military batteries need to be designed with long life and high reliability in mind. They could also experience a wide range of environmental conditions [4]. |
| A new approach has been described to estimate the SOC of lead acid battery using Radial basis function based learning method. The proposed method considers battery non linearity due to discharge rate, with temperature and corrects itself for aging and other variations of the battery characteristics to estimate SOC. Experimental results suggest that the proposed method gave excellent prediction of SOC assuming that the initial charging state of battery is known and is able to learn performance variation. The proposed algorithm can further be extended to include factors such as incomplete charging and interrupted discharging [5]. |
| A new estimation method of the SOC on the lead acid battery is proposed. Using an electric circuit model of the battery, it is shown how the open circuit voltage (which is directly related to the SOC) can be estimated based on the terminal voltage and current measurements provided there is sufficient variation in the battery current. A modified Thevenin equivalent circuit model given was used to represent the lead-acid battery. Treatment of nonlinear time varying model to a linear time varying model is done with an unknown constant parameter. Conditions were found on the battery current that ensure the observability Gramian of the system is full rank so that the initial state of the system can be found using the inverse of the systemGramian [6]. |
| The storage of energy in batteries is a cause of the failure and loss of reliability in PV systems. The two general lead acid battery models and their agreement with experimental data were reviewed. In order to validate these models, the behavior of different battery cycling currents has been simulated. The results obtained have been compared to real data. The CIEMAT model presents a good performance compared to Monegon’s model. An experimental study was presented for a different type of batteries. The two models used for comparison with experimental data are general and can be applied for wide range of lead acid batteries. The Monegon model was analysed and found that the equation of charge and discharge does not reproduce the experimental curves. Probably, the value of parameters was fitted for to another type of battery and different operational conditions. The term included in Monegon model for the overcharge does not reproduce these effects and values of RMSE indicate the deviation. The CIEMAT model presents a good performance to represent dynamic and complex battery operation. This is, in contrast to Monegon’s model; which presents significant limitations with respect to charging process. In this way, other results could be evaluated considering parameter variations effects in the life of battery. The aging model describing life time of a battery is useful for an economic analysis [7]. |
| Renewable energy sources, such as wind energy and photovoltaic (PV) energy, are widely used as stand-alone power systems supplying different electrical loads in rural and remote areas. These sources are of intermittent nature and, therefore, the stand alone power systems should include storage battery banks. The storage battery banks improve the reliability of these systems because the excess energy is stored in the battery bank, and this energy is delivered to the load when the solar or wind energy is not available or not sufficient. With respect to reliability and cost of standalone PV power systems, storage batteries represent main and important components. Even a battery block represents only 8% of the initial cost of a new PV system; it represents 23% of the total system cost when considering the replacement of batteries during the total life time of the system (20 years). |
STORAGE BATTERY TYPES |
| A rechargeable battery or storage battery is a group of one or more electrochemical cells. They are known as secondary cells because their electrochemical reactions are electrically reversible. Rechargeable batteries come in many different shapes and sizes [8]. |
| A. Lead-Acid batteries |
| The charge-discharge of a lead-acid battery process is essentially reversible, the system does not suffer from deleterious chemical action, and while its energy density and specific energy are low, the lead-acid battery performs reliably over a wide temperature range. A key factor for its popularity and dominant position is its low cost with good performance and cycle-life. Its limitations relatively low cycle life (50-500) and difficult to manufacture in very small size ( inmAh) [6]. |
| B. Alkaline secondary batteries |
| Most of the other conventional types of secondary batteries use an aqueous alkaline solution (KOH or NaOH) as the electrolyte. Electrode materials are less reactive with alkaline electrolytes than with acid electrolytes. Furthermore, the charge-discharge mechanism in the alkaline electrolyte involves only the transport of oxygen or hydroxy ions from one electrode to the other; hence the composition or concentration of the electrolyte does not change during charge and discharge. |
| C. Nickel-Cadmium batteries |
| The nickel-cadmium secondary battery is the most popular alkaline secondary battery and is available in several cell designs and in a wide range of sizes. A key factor for it is long cycle life, excellent long-term storage, low maintenance and good charge retention. Its limitations refer to low energy density and higher cost than lead-acid batteries. |
| D. Nickel-Iron batteries |
| It was used in materials-handling trucks, mining and underground vehicles, railroad and rapid-transit cars, and in stationary applications. The main advantages of the nickel-iron battery, with major cell components of nickel-plated steel, are extremely rugged construction, long life and durability. Its limitations, namely, low specific energy, poor charge retention, and poor low-temperature performance, and its high cost of manufacture compared with the lead-acid battery led to a decline in usage [9] |
LEAD - ACID BATTERY CHARACTERISTICS |
| The nominal voltage of a lead - acid cell is 2V, while the lower and upper limits of discharging and charging open circuit voltage at 25 °C cell temperature are 1.75 and 2.4V (resp.), which corresponds to 10.5 and 14.4V for a 12 V battery (resp.). Lead (Pb), lead oxide (PbO2), and sulfuric acid (H2SO4) are the negative electrode, positive electrode, and electrolyte of lead acid batteries, respectively. The overall battery reaction is shown in Eq. (1). |
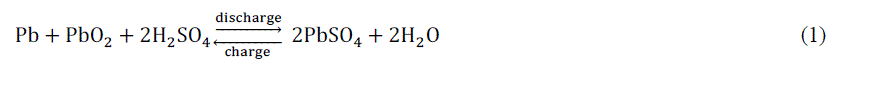 |
| A. Storage capacity and efficiency |
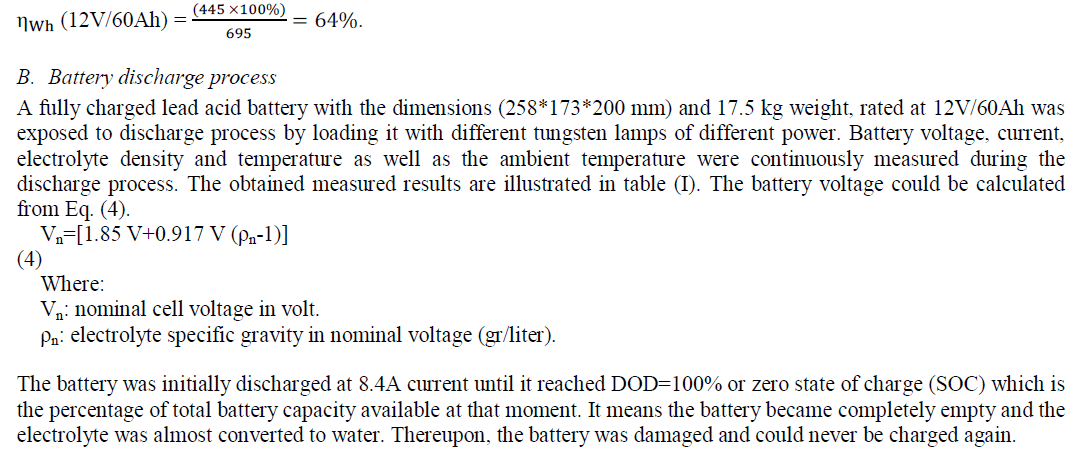
| The energy storage of a battery is expressed by its ampere hour capacity (Ah) or its watt hour capacity (Wh). Ah is current in ampere, multiplied by the time in hours, during which the current flows from the battery [10]. For example, a 12V battery rated at 60Ah over 20 hours can deliver 3A per hour for 20 hours (C20). The ampere hour capacity (AhC) is the time integral of the product of discharge current from fully charge (The Depth of Discharge (DOD=0) is a measure of how deeply a battery is discharged) to (DOD= 60%). (12*60Ah) is equivalent to (720Wh=0.72kWh) of energy which is known as watt hour capacity. The watt – hour capacity (WhC) or energy capacity is the time integral of the product of discharge current and voltage from fully charge (DOD = zero) to (DOD= 60%). The ampere hour efficiency of a battery (ηAh) is the ratio of the number of ampere hours delivered during discharge to that needed to return to its original condition, Eq. (2). (ηWh) is the ratio of the number of watt hours delivered (energy delivered) during discharge to that needed to return it to its original condition, Eq. (3). |
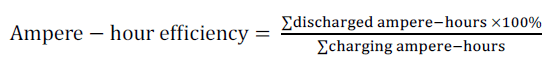 (2) (2) |
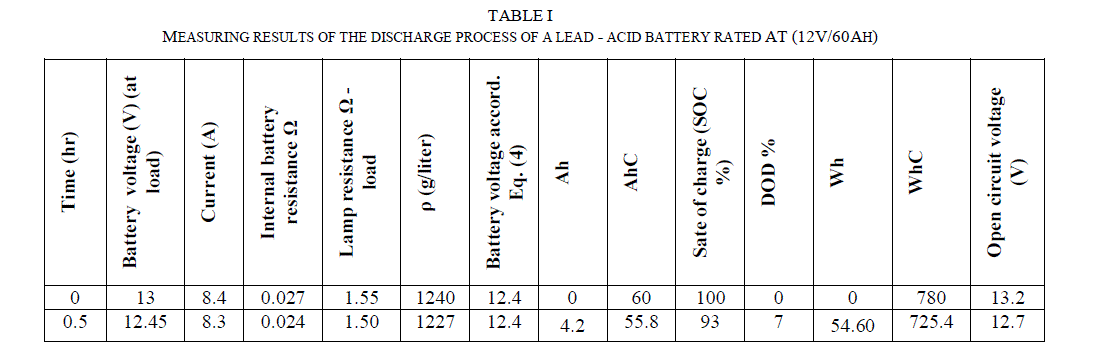
| The summation of discharge ampere – hours = 36 at DOD = 60% (see table (1)) and the summation of charge ampere – hours = 44.4 at DOD = 60% (see table (2)). |
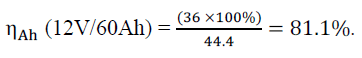 |
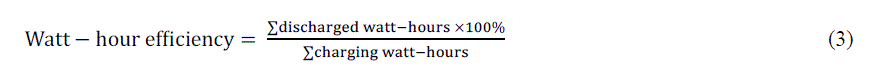 |
| The summation of discharge watt – hours = 445 at DOD = 60% (see table (I)) and the summation of charge watt – hours = 695 at DOD = 60% (see table (II)). |
 |
 |
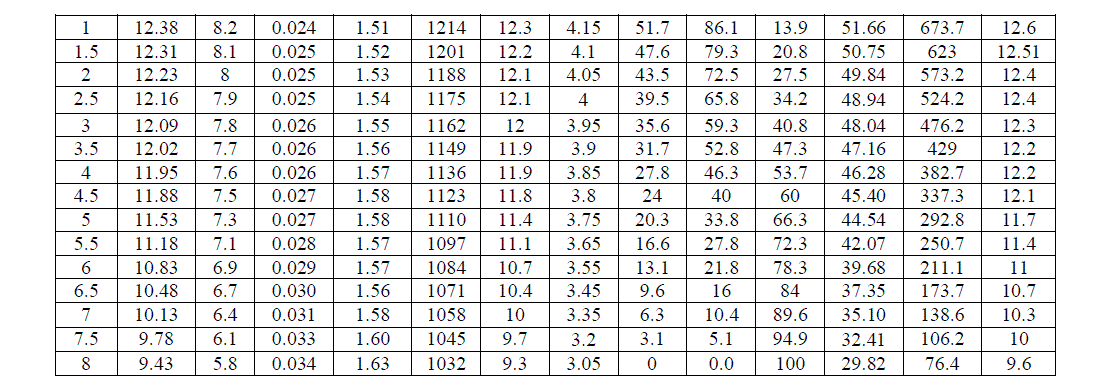 |
| Table (I) shows that the electrolyte specific gravity became 1032 gr/liter which is very closed to water density which is (1000 gr/liter). At this condition, the battery voltage dropped to 9.43V while the open circuit voltage of the battery dropped to 9.63V. Therefore, the battery should never be discharged under 10.8V. |
| C. Battery charging process |
| The constant voltage (constant potential) charge method applies a constant voltage to the battery which is greater than the battery voltage. The battery will be gradually charged and the charge current will decrease automatically according to the state of charge represented in table (II). |
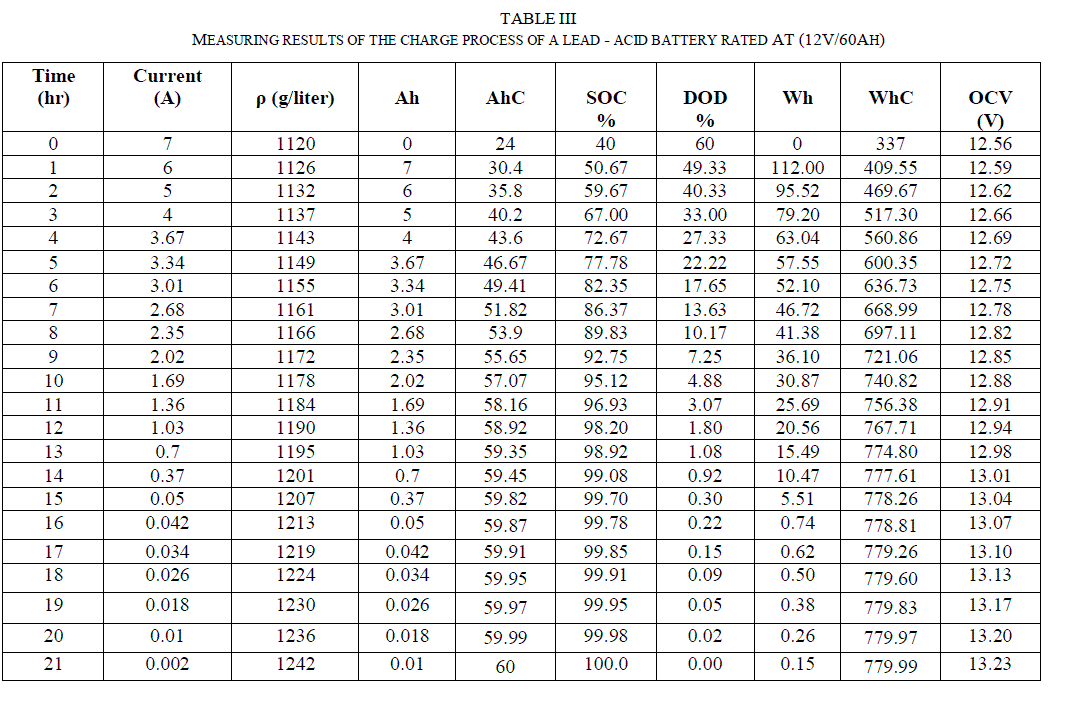 |
| Table (II) illustrated that the charging voltage value applied to the battery was 14.4V and was hold constant during the whole process. |
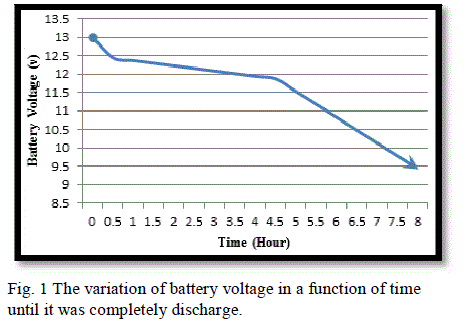 |
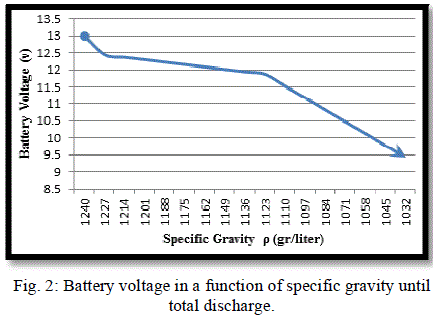 |
| Fig. (1) and Fig. (2) Show the battery behaviour under the mentioned damaging discharge. In this case (SOC = 0%), (DOD = 100%), (AhC = 0) and (VOC = 9.63V) and the time of discharged was (8 hr), this graphs show the limitation of the battery voltage that we should not exceeded in discharge process. |
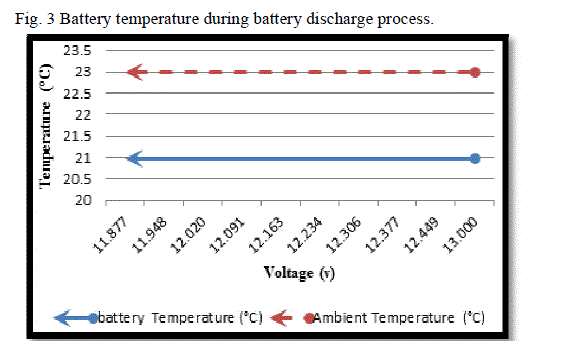 |
| Considering the curve in Fig. (3), we see that the temperature of battery does not differ during discharge. |
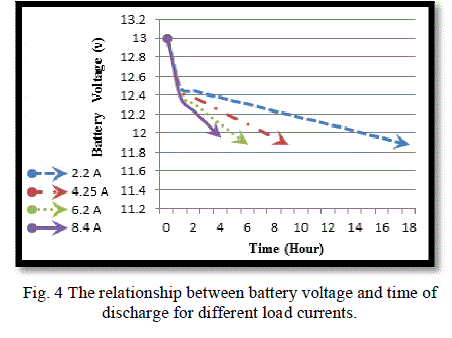 |
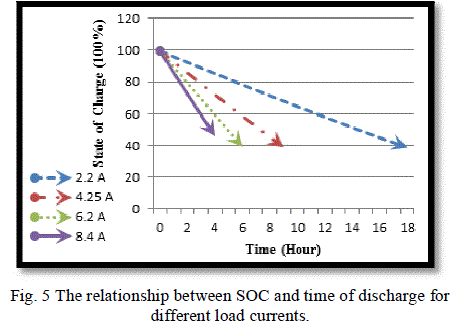 |
| Fig. (4) Shows the time needed to discharge the battery under different loads. As the current increases, the time of the discharge decreases and the battery becomes empty or reaches to (DOD = 60%) faster and the same is true for SOC which reaches (40%) faster at higher current, as shown in Fig. (5).this graphs show the limitation of the battery voltage that we should not exceeded in discharge process and the speed of discharge for different load currents. |
| Table (I) shows that the specific gravity at (DOD = 60%) is 1123 gr/litre and after few days is 1120 gr/litre as it is shown in table (II) before the process of charge due to the self of discharge, this means that the battery loses the capacity slowly when it is not used or stored. At 21°C the self of discharge rate for lead acid battery approximately from 15% to 30% per month for starting-lighting-ignition (SLI) [4]. |
 |
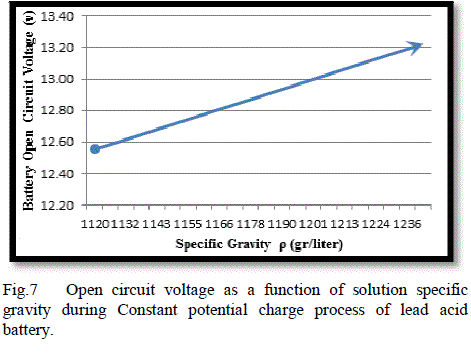 |
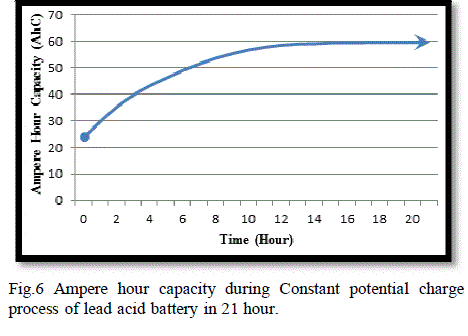
| The current delivered from the voltage source to the battery decreases and the AhC increase as it illustrated in Fig. (6). Fig. (7) Illustrate the variation of open circuit voltage in a function of specific gravity.this graphs show the limitation of the battery voltage of the charge process and the speed of charging. |
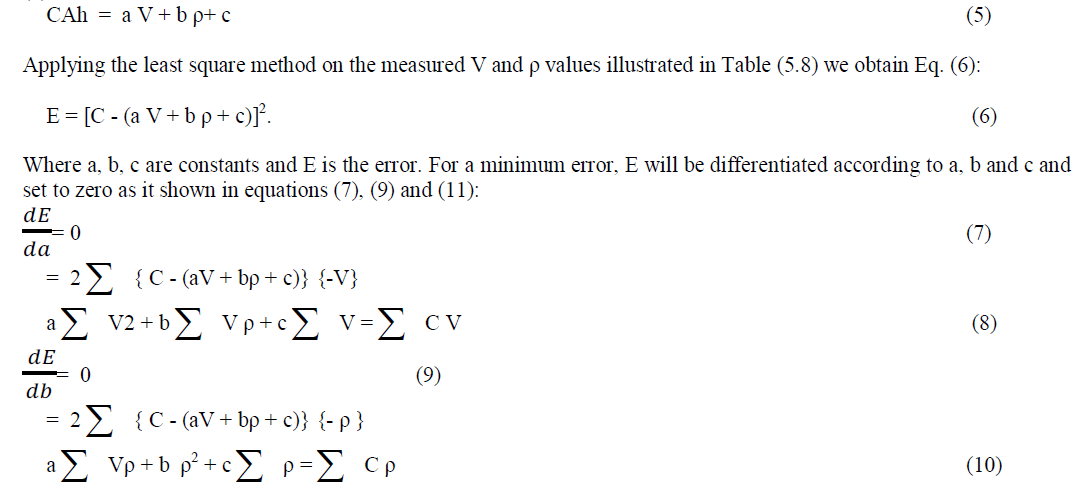
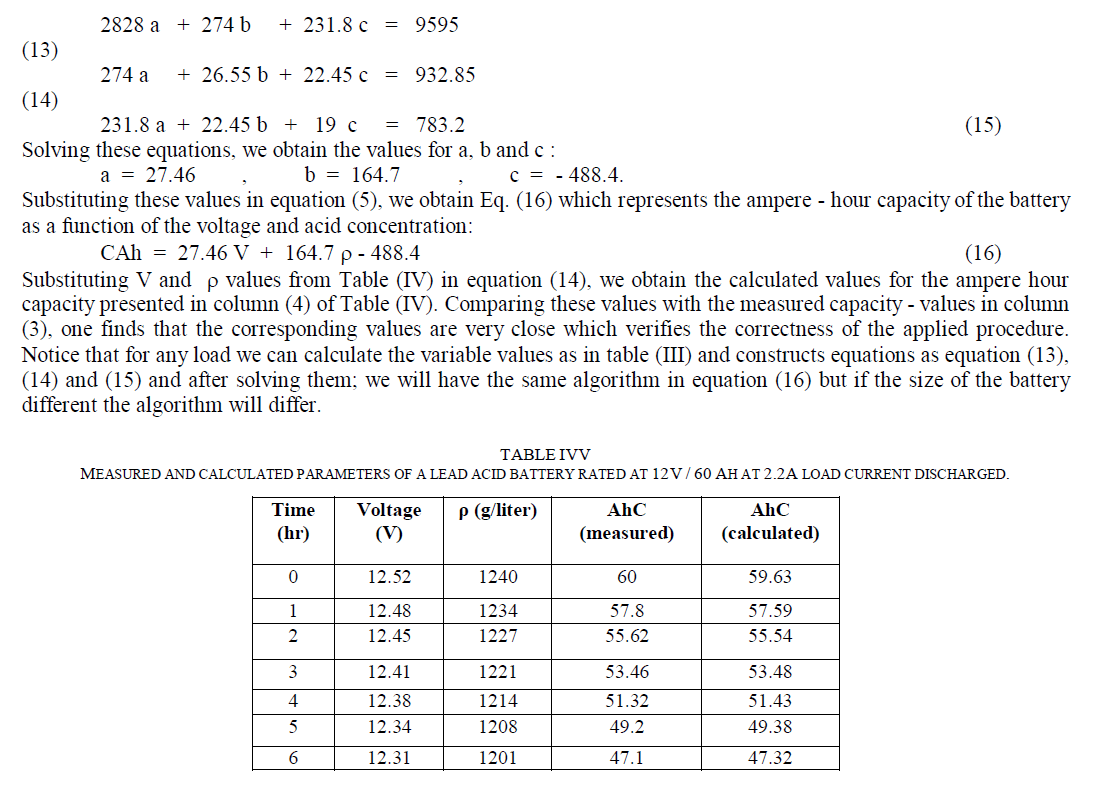
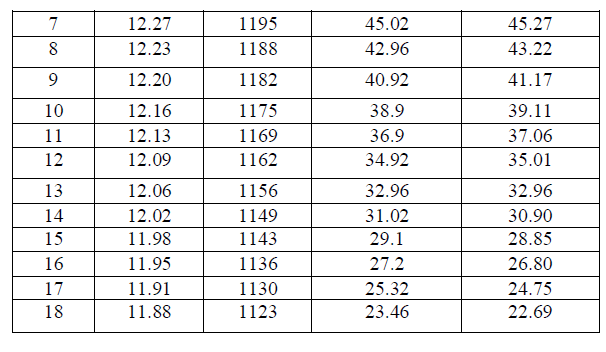
| Developing an Algorithm for Determining the Battery – Ah CapacityAt quasi-constant acid temperature, the ampere - hour capacity of a battery (C) can be represented as a linear function of voltage and acid concentration according to Eq. (5). |
 |
 |
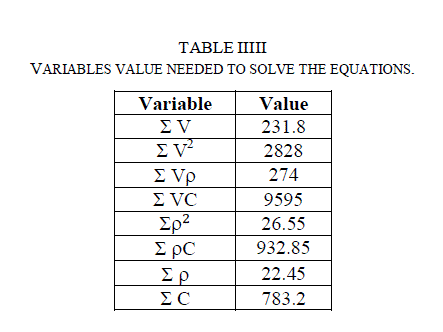
| Table (III) shows the values of calculations of V2, Vρ, VC, ρ 2 and ρ C and their summations from Table (IV). Substituting the corresponding values in equations (8), (10) and (12), we obtain the following three equations with three unknown constants: |
 |
 |
 |
CONCLUSIONS |
| Lead - acid storage batteries are usually used in small and large PV power systems operating in stand-alone mode. Selection of battery type and capacity are important factors to realize an efficient PV system. Battery voltage in function of electrolyte temperature, depth of discharge and specific gravity as well as the battery capacity in function of discharge current have to be given special consideration when evaluating or designing storage batteries for PV power systems. Battery voltage and specific gravity together is the key for determining the ampere - hour capacity of a battery and the stored energy in it. Measuring them at the same time and substituting their values in the developed algorithm, result the capacity of the battery in Ah. This algorithm enables knowing the battery state of charge within a PV power system and to perform accordingly in setting the battery charger control limits correctly, which consequently elongate the life time of the battery and enhance the overall PV system performance and economics. The performed tests have proved that the battery ampere hour efficiency is considerably higher than the watt hour efficiency (ηAh = 81.1% and ηWh = 64) which justify the consideration of ηWh more than ηAh when designing or evaluating of storage batteries for PV power systems. |
References |
|