ISSN:2321-6212
ISSN:2321-6212
A.El Hamidi*, E.Elmahboub, A.El Hichou, A.Almaggoussi
Department of Optoelectronic Materials, Cadi Ayyad University, Marrakech, Morocco
Received:27-Jan-2022, Manuscript No. JOMS-22-52516; Editor assigned: 29-Jan-2022, PreQC No. JOMS -22-52516(PQ); Reviewed: 10-Feb-2022, QC No. JOMS -22-52044; Revised: 12-Feb-2022, Manuscript No. JOMS -22-52516(R); Published: 19- Feb-2022, DOI: 10.4172/2321-6212.10.2.002.
Visit for more related articles at Research & Reviews: Journal of Material Sciences
The aim of this work is to study the effect of the solvent on the structural, morphological and optical properties of Mg doped Zinc Oxide (MZO) thin films. The results of XRD analysis revealed that 2-methoxyethanol solvent grants MZO samples a preferential orientation following the (002) plan taking a maximum value at 2% Mg. While, the samples prepared by methanol solvent shows no preferential orientation. SEM analysis corroborated that elaborating with 2-methoxyethanol attribute an orderly distribution to MZO crystallites. The optical characterization showed that the transmittance of MZO thin films reached a maximum value of 90% for (2% to 3%) Mg with the refractive index showed the lowest value of (1.46). The elaboration with methanol solvent exhibit that the transmittance reaches a maximum value of 80% at 4% Mg, and a minimum of the refractive index with a value of 1.96.
Sol-gel; Mg doped ZnO; Methanol; 2-Methoxyethanol; Optical properties; Thin film
For many years, the main applications of zinc oxide have been used in the chemical and pharmaceutical industry. Nowadays, new avenues of research in optoelectronics are arousing a great interest of this material due to its multiple properties. ZnO is a semiconductor with a large band gap (~3.37 eV), high bond energy (60 meV), and it is transparent in the visible and in the near infrared range. It is considered as a "twin" of GaN material [1], which makes it interesting for potential applications in the fields of photovoltaics [2], light-emitting diodes for lighting [3], Transparent Conductive Oxides (TCO) [4], photonics or sensors [5]. It also has specials property from the II-VI family: hardness, exciton stability, piezoelectricity, thermochromicity [6].
For the design and realization of ZnO- based devices, one of the major problems for having an efficient TCO is to find an adequate doping which offers the TCO material both a high conductivity and a high transmittance. A concentration of carriers with a value of 1019 cm-3 or more, and band gap energy greater than 3.3 eV are generally required for a high conductivity and transmittance [7]. Fortunately, ZnO thin films are a promising alternative to the commonly used ITO, which are non-toxic and less expensive than ITO [8]. One of the major challenges of optimizing the optical properties of ZnO is the incorporation of doping ions into the ZnO lattice. It was reported by many researchers that the optical properties of ZnO can be modified by doping ZnO with a group of II elements [9,10].
In this study, Mg-doped ZnO thin films are elaborated by two different solvents named 2-methoxyethanol and methanol in order to realize an enhancing of the optical properties of TCO devices with affecting the lattice parameters, under the influence of difference electronegativity achieved by replacing Zn2+ ions by Mg2+. The variation in the optical properties with the change of the solvent mainly depends on two important parameters which are the dielectric constant and the boiling temperature.
The samples un doped and Mg doped ZnO thin films were prepared on glass using a sol gel process followed by spin coating. A homogenous solution was prepared by dissolving zinc acetate dehydrate [Zn (CH3OO)2.2H2O] as a starting materiel and the 2-methoxyethanol (C3H8O2) was used as the solvent with Mono Ethanol Amine (MEA) as the stabilizer. The zinc precursor solution was prepared with the MEA stabilizer at ratio of 1:1. The Mg content was taken at 0%,1%,2%,3%,4% and 5% using magnesium acetate [Mg(CH3COO)2.4H2O] as a doping element. The same procedure is redone, but with the methanol solvent in order to study the effect of the solvent on the optical properties of these samples. Each film layer was deposited using a spin coater operating at 3000 rpm for 30 seconds, after cleaning ultrasonically the glass substrates in acetone and rinsed in deionized water. After, the films were air dried at 150°C. This process of drying was repeated 10 times for each film. Finally, the films were annealed at 500°C for one hour. Films were analysed using an X-ray diffraction (XRD, D/Max-2400) to analyse their crystal structure. A scanning electron microscope (SEM, JSM-6701F) was used to explore the morphological surface. The transmittance spectra of the UV-visible light passing through the films was measured by a UV-visible spectrophotometer (Lambda35 UV/VIS) in order to study the optical properties of the studied samples.
Structural and morphological characterizations
X-ray diffraction was used to determine the crystal’s structure, the crystal’s orientation, and the crystal’s size. The Figure 1 shows that the samples of ZnO doped with Mg elaborated with two different solvents namely 2-methoxyethanoland methanol crystalized with wurtzite structure and exhibit tree peaks characteristic of ZnO correspond to (100), (002), and (101) peaks. Except that, the samples prepared by 2-methoxy-ethanolshowed more intense peaks with the appearance of the secondary peaks compared to those synthesized with the methanol solvent. To analyse the orientation of the crystallites for each solvent, the Texture Coefficient (TC ) (hkl) for each diffraction peak was calculated following this equation [11].
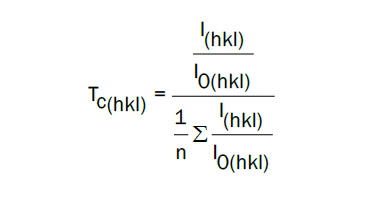
0% Mg = ZO, 1%Mg =M1ZO, 2% Mg =M2ZO, 3% Mg =M3ZO, 4% Mg =M4ZO, and 5% Mg =M5ZO
We note that the texture coefficient of all the samples prepared by methanol solvent is around 1, which confirms that the crystallites have a disordered distribution. Since the preparation with 2-methoxyethanol gave a texture coefficient greater than 2 for 2% Mg. We can conclude that the elaboration with 2-methoxyethanol gives the crystallites a better crystallinity compared to the samples prepared by methanol. Indeed, the methanol has a boiling point equal to 64.7°C it evaporates quickly leaving a disorder in the distribution of crystallites, while 2-methoxyethanol has a larger boiling point equal to 126.4°C. In this case, evaporation takes place slowly, giving the crystallites enough time to have a preferred orientation. Bekkari et al. [12] indicated that in the solvents with a low boiling point, the evaporation is faster and forces the material to develop in other directions and generally behaves like an amorphous material.
The calculated average crystallite size was obtained from the Scherrer relation associated at the (002) peak for each solvent (Tables 1 and 2).
| Mg concentration | 0% | 1% | 2% | 3% | 4% | 5% |
|---|---|---|---|---|---|---|
| TC(002)2-methoxyethanol | 1.28 | 1.47 | 2.16 | 1.92 | 0.71 | 0.93 |
| TC (002) methanol | - | 0.891 | 1.11 | 0.96 | 1.36 | 1.11 |
Table 1. Texture Coefficient of Mg doped ZnO thin films deposited on glass substrate by sol gel elaborated with two different solvents: 2-methoxyethanol and methanol.
| Mg concentration | 0%Mg | 1%Mg | 2%Mg | 3%Mg | 4%Mg | 5%Mg |
|---|---|---|---|---|---|---|
| D(nm) (2-methoxyethanol) | 33.52 | 65.6 | 80.70 | 75.5 | 35.66 | 35.66 |
| D(nm) (methanol) | - | 35.6 | 42.91 | 53.679 | 53.42 | 35.82 |
Table 2. Crystallite size of Mg doped ZnO thin films deposited on glass substrate by sol gel elaborated with two different solvents: 2-methoxyethanol and methanol.
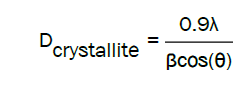
The crystallites size for samples prepared with the 2-methoxyethanol reaches a maximum value of 80, 70 nm at 2% Mg and it begins to decrease. For the samples elaborated with the methanol solvent, the crystallite size varied slightly between 35 nm and 53 nm, which in all cases remains much smaller than those obtained with 2-methoxyethanol. This difference in size can be attributed to the difference in dielectric constant and in the boiling point of the two solvents. Indeed, the values of these magnitudes are (32.35; 64.7°C) for methanol and (16.93; 126.4°C) for 2- methoxyethanol. In this context, Joshi et al. [13] have shown that the larger the dielectric constant is, the smaller the crystallite size is.
The lattice parameters a and c is calculated by the equation [30]:
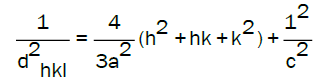
In the case of the 2-metoxyethanol, the lattice parameters a and c decrease simultaneously with increasing Mg concentration up to 3% Mg, then they increase (Figure 2). This result may be due to the occupation of the substitutional sites by Mg atoms for concentrations ranging from (1% to 3%) Mg, under the influence of a large difference in the electronegativity between the Zn2+ (1.65) and Mg2+ (1.31) ions. While, the re-increase results of occupying the substitutional sites by Mg atoms [14]. On the other hand, the parameter "c" in the case of methanol solvent undergoes a remarkable variation and reaches a minimum value of 3% Mg. Conversely, the parameter “a” increases and reaches a maximum value at the same percentage of doping. This phenomenon can be explained by the presence of an intern stress during the insertion of Mg into the structure of ZnO. Indeed, the samples, produced by methanol, with a low boiling temperature, have a poor crystallinity and are more likely to contain more constraints than the ones elaborated by 2-methoxy-ethanol. Table 3 shows that these constraints reach their maximum at 3% Mg with a significant value of 5.4 GPa to compare with 2.4 GPa for ZnO: Mg produced by 2-methoxy-ethanol, which causes an extremum of lattice parameters “a” and “c”.
Some additional characterizations SEM (Scanning Electron Microscopy) are essential in order to obtain more detailed and precise information on the size, shape or distribution of the grains. The SEM images of the MZO samples prepared by 2-methoxy-ethanol show that the samples had a nano scale crystalline microstructure with a uniform and dense distribution. It was also observed that the grain size increases from the concentration 1% Mg to 3% Mg. On the other hand, the MZO samples prepared by methanol present nanowires of different orientations and different shapes, presenting the agglomerations of grains. This difference in morphology can be attributed, as has already been reported, to the difference in the dielectric constant and the boiling point between methanol and 2-methoxyethanol. In fact, the thermal decomposition of zinc acetate form ZnO nuclei is more spontaneous in the solvent which has a high dielectric constant [15].
| Mg concentration | 0%Mg | 1%Mg | 2%Mg | 3%Mg | 4%Mg | 5%Mg |
|---|---|---|---|---|---|---|
| σ(GPa)(2-methoxyethanol) | -0.33 | 1.55 | 2.33 | 2.42 | 1.38 | 0.95 |
| σ(GPa)(methanol) | - | 0.4 | 3.95 | 5.4 | 3 | 0.72 |
Table 3. Stress of Mg doped ZnO thin films deposited on glass substrate by sol gel elaborated with two different solvents: 2-methoxyethanol and methanol.
Optical properties
The Figures 3 and 4 shows the optical transmission spectra of MZO films at a various percentage prepared with two different solvents in the range of 300-1000 nm. For the methanol solvent, the transmittance of the samples MZO decreases as soon as the Mg is introduced then increases when we reach a percentage of 4% Mg with a value of 80%. There is an opposite phenomenon to that obtained using 2-methoxyethanol. Indeed, with the latter, the transmittance increases and reaches a maximum value greater from 90% at 3% Mg corresponding to a high texture coefficient (1.96). So, in the case of elaborating with methanol solvent, the M4ZO sample has a high texture coefficient as well (1.26).Consequently, we think that the evolution of transmittance is linked to the crystallinity, in particular to the preferential orientation of the samples according to the plan (002).
1%Mg =M
ZO, 3% Mg =M3ZO, and 5% Mg = M5ZO
From the transmittance spectra, the optical gap Eg was deduced of the MZO films, by applying the following equation:
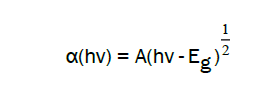
Where α is the absorption coefficient, and A is a constant. The figure 5 shows the evolution of optical band gap in function of Mg concentration for methanol and 2-methoxyethanol solvents. For the methanol, the gap increases and reaches a maximum value at 4% Mg with a value of 3.33 eV, while, it reaches the maximum value in the case of 2- methoxy-ethanol at 3% Mg with 3.35 eV value. It is noted that the energy of the optical band gap varies practically in the same way for the two solvents with a displacement of the maximum at 4%.
The refractive index n (λ) is related to reflectance, transmittance, and extinction coefficient k(λ) by Gautam SK, et al [16].
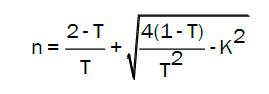
Where k is the extinction coefficient (k = αλ /4π) and T is the transmittance.
Figures 6 and 7 show the refractive index n (λ) and extinction coefficient k (λ) of MZO thin films at different percentage of Mg doping prepared with two different solvents in the range of 380–1500 nm. These optical constants exhibitan exponential decrease with increasing (λ), therefore, we have grouped in Tables 4 and 5 their values for λ=600 nm, in order to cipher their evolution with Mg concentration. These results show that the refractive index increases as soon as the Mg is introduced, taking values greater than n=2 recorded for undoped ZnO, then decreasing at 4% Mg taking a minimum value of 1.96. The increase can be caused by a disorder in the structure, changes in stoichiometry or by the creation of internal tension caused by the increase in polarizability [17,18]. This behavior contrasts with MZOs samples prepared by 2-methoxyethanol, which make refractive index take a minimum going up to 1.46 for 3% Mg. This proves that the elaboration of MZO thin films with 2-methoxyethanol solvent gives the samples a better transparency compared to those prepared by methanol solvent. It can be concluded that the choice of solvent is able to modify the optical properties of ZnO doped Mg. The extinction coefficient takes high values in the case of methanol for the percentages 1%, 2% Mg and 3% Mg compared to the undoped ZnO, and then it decreases taking a minimum value of 0.04 at 4% Mg. This increase is mainly due to the increase of absorption. While with the 2-methoxyethanol, k (λ=600 nm) achieved its minimum value of 0,022 at 3% Mg. It can be concluded that the best TCO obtained, is the one of M3ZO and M2ZO prepared by 2-methoxyethanol solvent.
| Mg concentration | 0% | 1% | 2% | 3% | 4% | 5% |
|---|---|---|---|---|---|---|
| n(2-methoxyethanol) | 2.04 | 1.56 | 1.55 | 1.46 | 1.9 | 2.66 |
| n(methanol) | - | 2.30 | 2.23 | 2.1 | 1.96 | 2.01 |
Table 4. Refractive index values determined for λ=600 nm of Mg doped ZnO thin films deposited on glass substrate by sol gel elaborated with two different solvents: 2-methoxyethanol and methanol.
| Mg concentration | 0% | 1% | 2% | 3% | 4% | 5% |
|---|---|---|---|---|---|---|
| k (10-2) (2-methoxyethanol) | 5 | 2.7 | 2.7 | 2.2 | 4 | 7 |
| k (10-2) (methanol) | - | 13 | 11 | 8.7 | 4 | 6 |
Table 5. Extinction coefficient values determined for λ = 600 nm of Mg doped ZnO thin films deposited on glass substrate by sol gel elaborated with two different solvents: 2-methoxyethanol and methanol.
Mg doped ZnO nano crystalline thin films has been prepared by sol-gel method with two different solvents named 2- methoxyethanol and methanol. Structural characterization using X-ray diffraction for the samples elaborated with 2- methoxyethanol has been reported a preferential orientation according to (002) plan taking a maximum value of 2% Mg. While, the samples prepared by methanol solvent show no preferential orientation, which confirms that the crystallites have a disordered distribution. These results are corroborated by SEM analysis. The optical studies exhibit that the elaboration of MZO samples highly improves the optical transmission, which reaches a maximum value of 90% for (2% to 3%) Mg accompanied by a minimum value of refractive index (1.46) lower than the undoped ZnO. Whereas, with the methanol solvent an opposite phenomenon to that obtained using 2-methoxyethanol is registered, reaching a maximum value of 80% at 4% Mg in transmittance, accompanied by a minimum value of refractive index (1.96).
[Crossref] [Google scholar] [Pubmed]