Keywords
|
| diabetes; self-monitoring of blood glucose; semi-invasive technique; optical reflectance method. |
INTRODUCTION
|
| The Embedded Technology is now in its prime and the wealth of knowledge available is mind-blowing. Embedded technology plays a major role in integrating the various functions associated with it. This needs to tie up the various sources of the department in a closed loop system. This proposal greatly reduces the manpower, saves time and operates efficiently without human interference. With the advent in technology, the existing systems are developed to have in built intelligence. There is no known cure for diabetes and patients rely on constant monitoring to maintain acceptable blood glucose levels. Depending on the type and severity of each case, therapy may include diet, exercise and other lifestyle changes, medication, and/or insulin injections. Insulin-dependent diabetics may need to inject themselves several times daily, with blood glucose testing before and/or after each meal. So far, most tests included actual blood tests, usually performed by pricking a finger and testing the blood using a portable device. |
| Diabetes mellitus is a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both [1]. Abnormally high levels of glucose can damage the small and large blood vessels, leading to: diabetic blindness, kidney disease, amputations of limbs, stroke, and heart disease [2]; also, excessive use of glucose-lowering medication such as insulin can cause hypoglycemia or abnormally low blood sugar. According to the Mexican National Health Information System there are more than 10 million people who are currently diagnosed with diabetes, of which 90% are type II diabetics, and it is the main cause of mortality in Mexico accounting for 13.6 percent of deaths in general [3]. Frequent monitoring of glucose concentration in diabetic patients is crucial for effective treatment because it can supply trend information that could help identify and prevent unwanted periods of hypo- and hyperglycemia [4]. Self monitoring of blood glucose is usually done in an invasive manner, which involves finger-stick testing which is painful to the patient and carries the risk of infection [5]. Recently, minimally invasive needle-based continuous glucose monitoring systems that can provide glucose measurements every 5 minutes or less have become available [4]. |
II. RESEARCH AND DEVELOPMENT OF NON-INVASIVE METHODS
|
| Several techniques such as diffuse reflectance spectroscopy, electrical impedance spectroscopy, Raman spectroscopy, among others, have been used for noninvasive glucose monitoring [6]; even though these studies were performed under different environments and clinical settings they can give a rough estimate of the sensitivity and reliability of current non-invasive glucose technology. These techniques present a Root Mean Square Error of reduction (RMSEP) that goes from 25 to 46 mg/dl [5,7,8,9,10,11,12,13,14]; which for a 126 mg/dl of Fasting Plasma Glucose level (FPG), which is considered a diagnostic criteria for diabetes [1], gives a relative concentration error higher than 5%. These studies rely on a single detection mechanism, but a combination of two or more detection mechanisms to reduce the error of prediction has not been investigated. |
| Near infrared (NIR) spectroscopy has been used to monitor changes of glucose concentration in tissue owing to the fact that a change of refractive index takes place in the extra-cellular fluid due to the presence of additional glucose, which causes a small change in the overall scattering properties of the tissue that can be detected by NIR spectroscopy [15]. It has previously been reported that glucose variations affect the electrical properties of cellular membranes [14]. This is due to specific reactions of blood and tissue cells to varying glucose concentrations, which changes the electrolyte balance across the membranes of blood and underlying tissue. These changes in the electrical properties of cellular membranes result in changes in the ac conductivity and tissue permittivity which can be measured using impedance spectroscopy [16]. |
III. OPTICAL ABSORPTION TECHNIQUES
|
| Optical absorption techniques for quantification of glucose are based on selective absorption of light by the molecule which is described by the Beer–Lambert law: |
| I=Io e-ε CL |
| Here Io is the intensity of incident optical radiation, I is the transmitted intensity, e is the molar extinction coefficient in (mol/L)-1 cm-1 and is dependent on wavelength, C is the molar concentration, and L is the pathlength in cm. Measurements are generally reported in absorbance, A=log(I0 /I), such that the absorbance of several species is additive. |
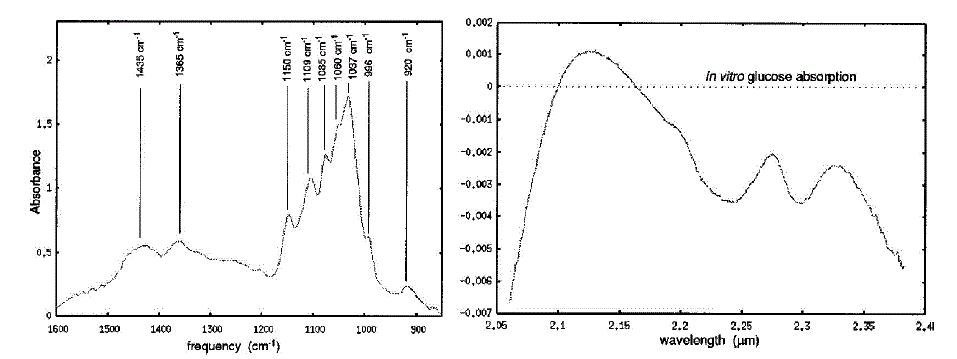
| Optical absorption spectroscopy for glucose quantification has generally been restricted to either the mid infrared MIR or the near-infrared NIR spectral region. Fig.1 shows examples of both MIR and NIR optical absorption spectra for aqueous glucose after water subtraction. The MIR region of the spectrum ranges from 2.5 to 50 um (4000–200 cm- 1), and it is in this region that absorption bands due to fundamental stretching and bending modes of the molecule may be seen. For this reason, spectroscopy in the MIR or ‘‘finger-print’’ region is extremely useful for spectral identification of compounds. However, the magnitude of background absorption bands due to solution constituents like water severely limits the path length which can be used in MIR transmission spectroscopy to a few hundred microns or less. The near-infrared region which lies between 2.0 and 2.5 mm has become increasingly popular for aqueous glucose measurements. This region contains a relative minimum in the water absorption spectrum and readily identifiable glucose peak information. Glucose sensing using near infrared spectroscopy is by no means a simple problem. Glucose absorption peaks whose magnitude is relatively small compared to a large aqueous background spectrum often yield low signal-to-noise measurements. NIR spectral measurements are further plagued by a lack of repeatability. Near infrared spectra are sensitive to a host of factors including temperature, pH, and scattering. Additionally, in vivo measurements may be susceptible to differences in skin pigmentation, hydration, blood flow, probe placement, and probe pressure. Finally, it should be noted that the NIR spectrum of glucose is very similar to that of other sugars including, in particular, fructose which is often used by diabetics as an alternative to glucose. Despite these difficulties, near infrared methods have demonstrated significant promise in becoming a viable technique for non invasive glucose sensing. For this reason we used near infrared reflectance technique for measuring blood glucose semi-invasively. |
IV. INSTRUMENTATION SCHEME FOR NON-INVASIVE GLUCOMETER
|
| The technology is based on the property of glucose to affect the scatter of light. Glucose changes the refractive index and hence the scattering properties of the organ (finger), leading to change in scattering coefficient, as a result concentration of glucose can be measured. |
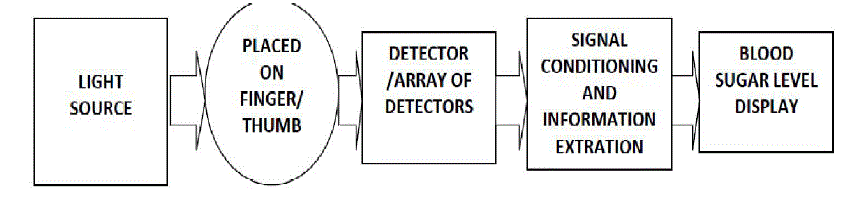
| In the proposed glucose sensing system, transmission mode is used in the design of probe. As in case of transmission mode the light traverses the thumb/finger, and typically encounter many more glucose molecules along their paths than in the reflection mode as a result increased sensitivity can be achieved corresponding to glucose concentration. However in this scheme fine calibration depending upon the skin thickness and pigmentation is considered. The generalized Instrumentation scheme for noninvasive blood glucose sensing system is given in Fig.2. |
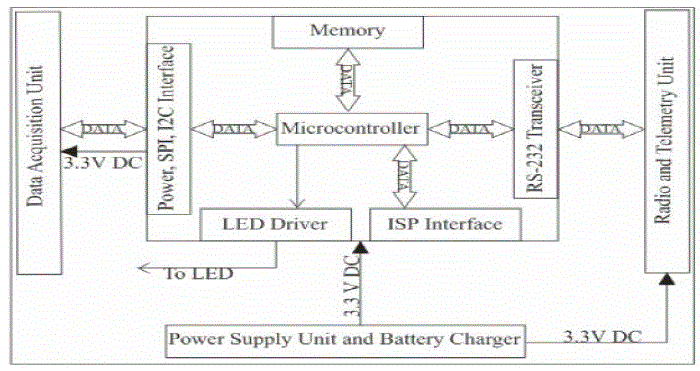
| This new technology allows for a non-invasive glucose-level blood testing. According to the company the new method is simple and accurate and may help people suffering from diabetes to live their lives in a slightly more comfortable manner without constantly worrying about being pricked with a needle. This proposed system introduces a non invasive type glucose measurement using non invasive type glucose sensor. The sensor is placed in human finger this sensor has two IR sensors the IR sensors pass infrared waves into the finger at different wavelength. According to the glucose level the IR waves get absorbed by the blood. The amplifier will amplify the reflected IR waves according to the obtained analog values the microcontroller will convert them into digital the digital values will be displayed in the PC. To interface PC with our microcontroller we need a level converter i.e., RS232 to TTL logic converter in PC we can view the values using VB.NET application. |
| Software Workflow in short: |
| 1. Power up the system. |
| 2. First detect the presence of finger via sensor & signal conditioning unit. |
| 3. Then trigger out the IR output signal via transistorized driver circuits. |
| 4. Wait for data input signal for logic change. |
| 5. Record time for change. |
| 6. Calibrate the time as required. |
| 7. Compare the time record for various sugar ranges. |
| 8. Send the message via GSM for abnormal ranges to concerned authorities. |
| 9. Send data to PC if required for data logging purposes. |
RESULTS
|
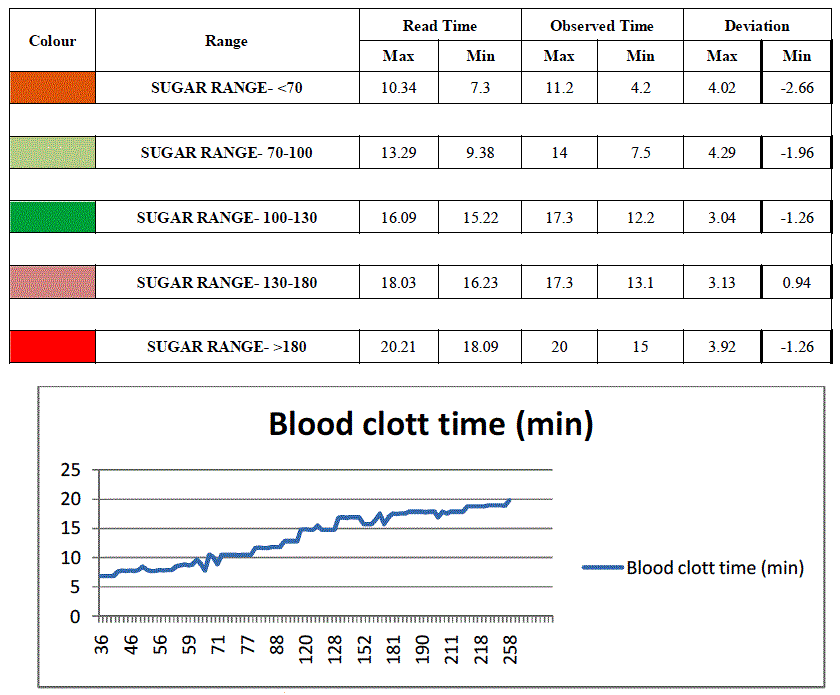
| The present work is based on the principle of relation between blood clotting time and blood sugar level. The blood sugar level is detected by monitoring the clotting time of blood. As the blood sugar level increases in blood the clotting time required will also be more. Hence the instrument is calibrated depending on the blood clotting parameter, the results as obtained from the present work are represented in the following table: |
VI. CONCLUSION AND FUTURE WORK
|
| The need for new glucose sensors in diabetes is now greater than ever. Although development of an acceptable, continuous & automatic glucose sensor has proven to be a substantial challenge, progress over the past several decades has defined sensor performance requirement & has focused development efforts on a limited group promising candidates. The advent of new glucose sensing technologies could facilitate fundamentally new approaches to the therapy of diabetes. Present paper demonstrates only fragments of a significant progress in its role study. Despite fulfilled significant scientific research work in this area there are a lot of blanks, problems to solve both in fundamental and practical issues of non invasive glucometer. Recent answers on puzzling questions mostly are limited via technical and methodological imperfections. Figuratively we are still at the beginning of the way, thorny and hard, and only productive cooperation of scientists may bring success. |
Figures at a glance
|
 |
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
Figure 5 |
|
| |
References
|
- American Diabetes Association, ‘Diagnosis and classification of diabetes mellitus’. Vol.31, Supplement 1, pp. S55-S60, 2008.
- G.L. Cot and R.J. Nichols, ’Glucose diagnostics’ Ch.18 in Biomedical Photonics Handbook, T. Vo-Dinh. Edition, CRC Press LLC, FL.3, pp. 1-17, 2003.
- O.S. Khalil, ‘Non Invasive glucose measurnment at the down of the new millinium’, Diabetes Technology Therauptics, Vol. 6, Issue 1, pp. 660-697, 2004.
- D.C. Klonoff ‘Continous glucose monitoring: roadmap for 21st century diabetes therapy’, Diabetes care, Vol. 28, Issue 5, pp.1231-1239 2005.
- S.f. Malin, T.L. Ruchiti, T.B.Blank, S.U. Thennadil and S.L. Monfre, ‘Noninvasive prediction of glucose by near-infrared diffuse reflectance spectroscopy’, Clinical Chemistry, Vol. 45, Issue 9, pp. 1651-1658, 1999 .
- A. Tura, A. Maran and G. Pacini, ‘Noninvasive glucose monitoring: Assesment of technologies and device according to quantitative criterion’, Journal of Diabetes research and Clinical practice, Vol. 77, Issue 6, pp. 16-40, 2007.
- M. Ries Robinson, R. Philip Eaton, David M. Haaland, Gary W. Koepp, Edward V. Thomas, Brian R. Stallard, and Paul L. Robinson, ‘Noninvasive Glucose Monitoring in Diabetic Patients: A preliminary evaluation’, Clinical Chemistry, Vol.38, Issue 9, pp. 1618-1622, 1992.
- K.U. Jagemann, C. Fischbacher, K. Danzer, U.A. Muller and B. Mertes, ‘Application of near-infrared spectroscopy for non invasive determination of blood/tissue glucose using neural network’, Physical Chemistry, Vol.191S, Issue 4 ,pp. 179- 190, 1995.
- H.M. Heise, “Technology for Non-Invasive Monitoring of Glucose” 18th Annual Internacional Conference of the IEEE Engineering in Medicine and Biology Society Amsterdam, M6: Minisymposium, pp.2159-2161, 1996.
- K. Danzer, Ch. Fischbacher, K.U. Jagemann, and K.J. Retchelt, IEEE LEOS Newsletter Vol. 12, Issue 2, pp. 9-11, 1998.
- T.B. Blank, T.L. Ruchti, S.F. Malin, and S.L. Monfre, IEEE LEOS Newsletter Vol. 13, Issue 5, pp. 9-12, 1999.
- G.W. Hopkins and G.R. Mauze, ‘In-vivo NIR diffusereflectance tissue spectroscopy of human subjects’ Technical report, HP laboratories Palo Alto, HPL-1999-13, 1999.
- S. Yeh, C.F. Hanna, and O.S. Khalil, ‘Monitoring blood glucose changes in cutaneous tissue by temperature modulated localized reflectance measurements’, Clinical Chemistry, Vol. 49, Issue 6, pp. 924-934, 2003.
- A. Caduff , M.S. Talary, M. Mueller, F. Dewarrat, J. Klisic, M. Donath, L. Heinemann, W. Stahel, ‘Noninvasive glucose monitoring in patients with Type 1 diabetes: A multisensory system combining sensors for dielectric and optical characterization of skin’ Biosensors and Bioelectronics, Vol. 24, Issue 5, pp.2778-2784, 2009.
- H.L. Liu, Y. Zhang, M. Kimura, and B. Chance, ‘Theoretical and Experimental Investigations on solute induced changes in optical properties in living tissues’ Spectroscopy and Diagnostics; E. Sevick–Muraca and D. Benaron, eds., Vol. 3 of OSA Trends in Optics and Photonics Series (Optical Society of America), paper CM3, 1996.
- A. Caduff, E. Hirt, Y. Feldman, Z. Ali, and L. Heinemann, ‘First human experiments with a novel noninvasive, non-optical continous glucose monitoring system’, Biosensors and Bioelectronics, Vol. 19, Issue 3, pp.209-217, 2003.
|