ISSN ONLINE(2319-8753)PRINT(2347-6710)
ISSN ONLINE(2319-8753)PRINT(2347-6710)
N.Mythili1 and N.Rajeswari2
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Innovative Research in Science, Engineering and Technology
Chemical packaging is one of the challenging fields of packaging because the materials used for packaging should be chemically resistant, inert, negligent gas transmission, non-reactive, nonflammable, non-corrosive, temperature resistant, and light resistant. Glass containers and plastic bottles are widely used for packing of chemicals. In this paper the study has been focused on hydrogen peroxide, one of the most important inorganic chemical compounds and it is widely used in cosmetics and healthcare industries as a cleansing and bleaching agent. In higher concentrations, it is unstable where as in lower concentrations, it is almost stable. Decomposition of hydrogen peroxide liberates oxygen, water and heat. The liberated oxygen will occupy in the headspace of the container which may cause the container to bulge. The aim of this work is to achieve breathable characteristics for a package of plastic bottle containing H₂O₂ of 12% concentration. A suitable experiment was identified to find out the volume of oxygen liberated by H₂O₂. During the primary level analysis, a method called micro perforation was identified as a solution for achieving breathable characteristics for effective storage of the product. The micro perforation was done in the closure (polypropylene) of the container. A head space analyzer was used to analyze the volume of oxygen accumulated in the headspace before and after the micro perforation. It has been found that the transmission of volume of the oxygen has been increased significantly.
Keywords |
| Bulging, Concentration, Decomposition, Microperforation, OTR, Storage |
INTRODUCTION |
| Hydrogen peroxide is inorganic peroxide which is a strong oxidizing agent. At various concentrations it is used as, antiseptic and cleaning agent (3%) , bleaching agent (3-30%) for bleaching pulp, paper, straw, leather, hair and above 90% it is used as monopropellant for rocket engines [1]. |
| Its value as an antiseptic is low, but the evolution of oxygen when it comes into contact with clotted blood helps to loosen dirt and assists in cleansing a wound. At higher concentrations, the decomposition of the peroxide is accompanied by the evolution of enough heat to convert the water to steam [1]. In this fashion, hydrogen peroxide is used as a monopropellant in rocket engines; the peroxide is passed over a silver mesh which catalyzes the decomposition and the resulting gaseous H2O and O2 products are ejected through a nozzle at high velocity propelling the rocket forward. Concentrated hydrogen peroxide can also be used as an oxidant with organic compounds, such as kerosene, in a bipropellant rocket engine. |
| The packaging of hydrogen peroxide solution will fall in the category of chemical packages. They are packaged in High Density Polyethylene (HDPE) containers especially of lower concentration (12%). At this concentration level, the liquid is almost stable. Once it is exposed to light, heat and other intrinsic factors like stabilizers, it starts decomposing followed by the release of water and oxygen [1], [9]. |
| H2O2 is an environmentally friendly chemical used for oxidation reactions, bleaching processes in pulp, paper and textile industries, waste water treatment, exhaust air treatment and various disinfection applications. H2O2 decomposes to yield only oxygen and water. H2O2 is one of the cleanest, most versatile chemicals available [9] - [11]. |
FACTORS AFFECTING STORAGE OF H2O2 |
| A. Effect of temperature |
| H2O2 is stable at most summer temperatures and will not freeze even at severe, cold winter temperatures (to - 52°C for 50% H2O2). However, if possible, H2O2 should be stored in roofed, fireproof rooms where it can be kept cool and protected from direct sunlight. It is very important that HâÃâÃâOâÃâÃâ should be protected against all types of contamination. With proper storage in the original containers or in tank installations, the solutions can be stored for long periods without noticeable losses in active OâÃâÃâ of less than 2 percent per year [2]. |
| B. Effect of pH |
| An increase in the temperature promotes the decomposition as well as a higher pH value. For optimum stability, the pH range of pure H2O2 is below 4.5. Above 5 pH, the decomposition increases sharply [2], [11]. Therefore, commercial solutions are generally adjusted to a pH value below 5. |
| C. Effect of stabilizers |
| The storage quality of hydrogen peroxide is negatively affected by impurities of every type even when some of these impurities (including stabilizers) are present in very low concentrations (ppm quantities). The decomposition can be induced homogeneously by dissolved ions with a catalytic effect. Heavy metals like iron, copper, manganese, nickel, and chromium are especially effective here. Hydrogen peroxide is also decomposed through the effect of light as well as by certain enzymes (catalase) [2], [11]. |
| As a result of the stabilizers, which are usually added to our commercial grades in ppm amounts, our hydrogen peroxide is protected against unavoidable impact during handling and has an excellent shelf life [2]. The loss of hydrogen peroxide can be minimized by normal handling and storage at low temperatures, also necessary precautionary measures should be taken. With normal handling and cool storage, and when they are observed, the losses of hydrogen peroxide are very slight even during extended periods (years) of storage. |
| D. Storage and handling of H2O2 |
| During storage and handling, in the presence of certain catalytically acting impurities, hydrogen peroxide will decompose exothermically to form water and oxygen. The stability of hydrogen peroxide solutions is influenced primarily by the temperature, the pH value, and the presence of impurities with a decomposing effect. Before few decades, these type of compounds are stored in thick walled tin containers and now plastic containers of Polyethylene grade is used because of its strength and non-reactive (for chemicals) properties [2], [5], [6]. |
| In chemical industries and laboratories, hydrogen peroxide of around 30% concentration will be used. In this case, certain preventive measures will be taken to avoid injury to the personnel. Table 1 describes the toxicity information of concentrated H2O2 of 33% concentration [12]. |
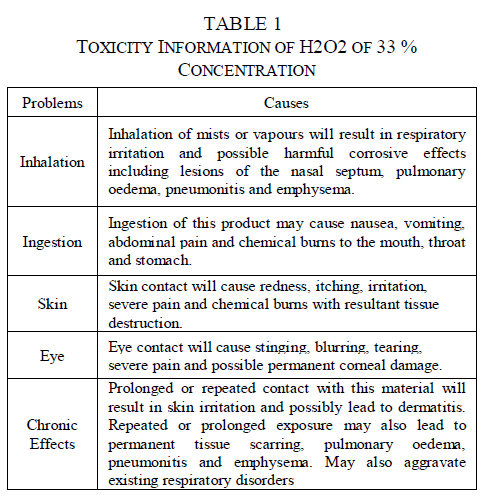 |
RATE OF DECOMPOSITION OF H2O2 |
| A. With Catalyst |
| Some set of experiments should be conducted to determine the rate of oxygen that is being released by the known concentration of H2O2 at standard conditions of temperature and pressure. Fig.1 shows the experimental setup which consists of a reaction vessel, O2 collection tank and a beaker to collect the displaced water. In this experiment, the known concentration of H2O2 is taken in the reaction vessel with added catalyst namely, ferric chloride (FeCl3). Due to the catalytic activity, the decomposition of H2O2 will be started and the released O2 molecules will be collected in the collection tank. Due to the pressure difference, water will be displaced out and collected in the beaker. The volume of oxygen released will be equal to the volume of water displaced [1]. This experiment should be conducted within 2-3 hours, unless the displaced water will be evaporated. This will be rectified in the next experiment. |
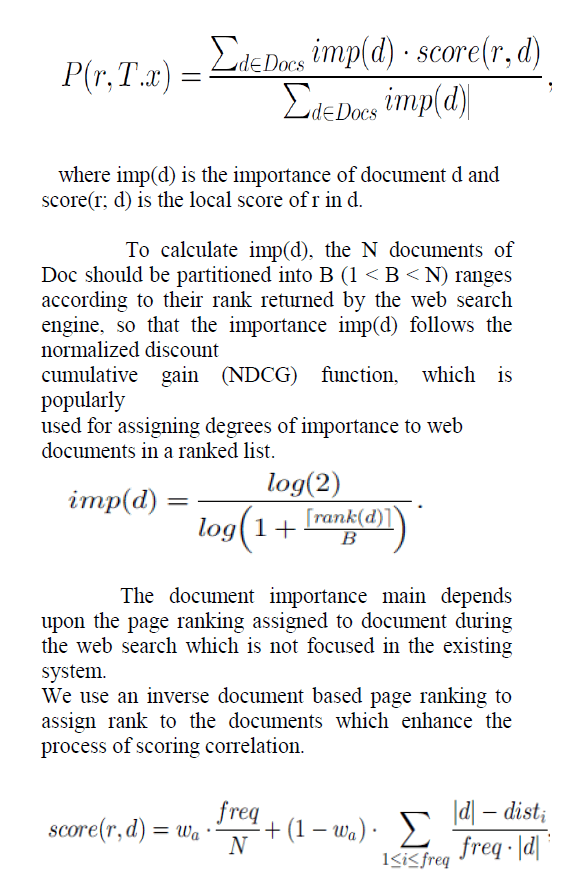
| With known concentration of H2O2, it is possible to compute the amount of O2 molecules released from the product by using the formula [1], |
 |
| B. Without Catalyst |
| Fig. 2 shows the experimental setup to determine the rate of decomposition of known concentration of H2O2 without the addition of catalyst [1]. This setup consists of a beaker, gas syringe and a rubber tubing connection. The released gas from H2O2 pushes the plunger in the syringe. Based on the displacement of the plunger, volume of gas occupied in the syringe can be measured. There was no air leakage in this system. Hence, accurate volume of oxygen can be determined. |
 |
METHODOLOGY |
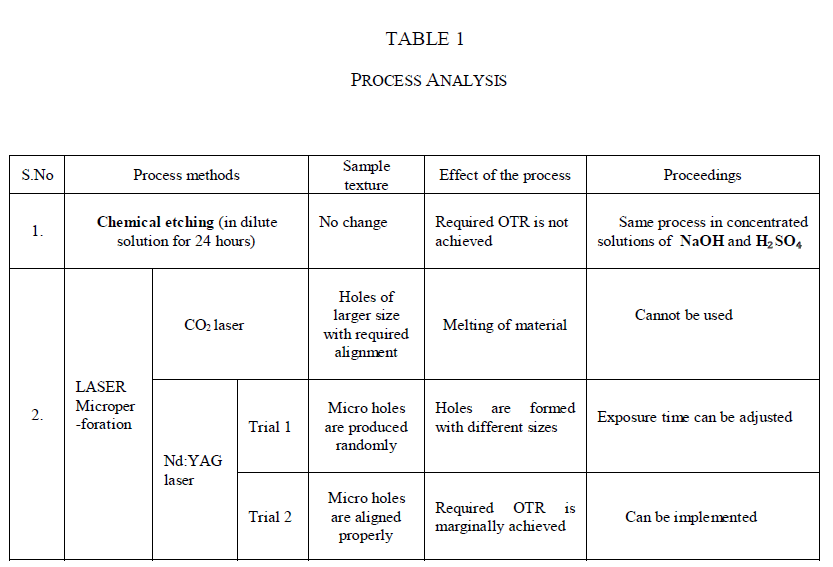
| Once the rate of decomposition of H2O2 was determined, oxygen transmission rate (OTR) was suitably set to release out the oxygen liberated [7]. Table 1 shows the process conducted and the results obtained. Chemical etching is a process in which the part to be etched is soaked in a chemical bath (NaOH/H2SO4) for 24 hours at ambient conditions. |
| Microperforation is a technique used in packaging materials to make the product to breathe out O2/CO2.This technique was already been employed in packaging of fresh fruits and vegetables in order to facilitate respiration at the time of post harvest [3], [4]. In the same aspect, H2O2 is liberating O2 and water at the time of decomposition. Anyhow, being a low concentration product, the rate of decomposition was considerably low. The oxygen thus liberated, during decomposition was occupied in the headspace of container (HDPE) causing bulging. A head space analyser was used to measure the volume of O2 in the headspace of the container [8]. Based on the measured volume of O2, the OTR of the closure material (PP) was tuned by making laser microperforation. |
| In this work, for laser microperforation, two types of industrial grade lasers were used namely, Carbon-dioxide (CO2) and Neodymium-doped Yttrium Aluminium Garnet (Nd:YAG) |
 |
CONCLUSION |
| Based on the process analysis results, the OTR of the closure material (PP) was marginally equal to the decomposition rate of the product (H2O2 of 12% concentration). The number of micro holes to be made depends upon the rate of decomposition of the product contained, storage conditions, surface area of the closure material, and volume of the product and transportation modes. Hence, the primary objective, that is, with the micro perforated plastic closure material; effective storage of the product was achieved in this work. |
References |
|