ISSN ONLINE(2278-8875) PRINT (2320-3765)
ISSN ONLINE(2278-8875) PRINT (2320-3765)
Karthikeyan S1, Rathna Priya K2, Ezhilarasi S3
|
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Advanced Research in Electrical, Electronics and Instrumentation Engineering
Fuel cell technologies have been identified as an important area in providing solutions to the problems of meeting increasing renewable energy demands. The Polymer Electrolyte membrane (PEM) fuel cell is commonly used to power vehicles. In this project, the modeling of proton exchange membrane fuel cell is developed. The controller used is a Proportional Integral and Derivative (PID) controller. The control technique performs the control of the output voltage by optimizing the input voltage. MATLAB / Simulink software is used for obtaining control parameters. A PID controller has been designed and the results indicate that the PID control strategy can improve the system’s performance significantly.
Keywords |
||||||||||
| Modeling, PID control, Fuel cell, Controller design | ||||||||||
INTRODUCTION |
||||||||||
| The first fuel cell was built in the year 1839 by Sir William Grove, a lawyer and scientist. The fuel cell which acts as a practical generator did not begin until the 1950's, when the U.S. space program chose fuel cells over nuclear power reactor and more expensive solar energy. Fuel cells supplied power for the Gemini spacecraft and Apollo spacecraft, and the fuel cell provides electricity and water for the space shuttle. A fuel cell is a device that converts the chemical energy from a fuel into electricity through a chemical reaction with oxygen or another oxidizing agent. The most commonly used fuel in fuel cells is hydrogen. Fuel cells can produce electricity continually for as long as inputs are supplied. | ||||||||||
ELECTROLYTE OF PEM |
||||||||||
| The electrolyte layer is essential for a fuel cell to work properly. In PEM fuel cells, the fuel travels to the catalyst layer and gets broken into protons (H+) and electrons. The electrons travel to the external circuit to power the load, and the hydrogen protons travel through the electrolyte until they reach the cathode to combine with oxygen to form water. The PEM fuel cell electrolyte must meet the following requirements in order for the fuel cell to work properly, | ||||||||||
| 1. High ionic conductivity. | ||||||||||
| 2. Present an adequate barrier to the reactants. | ||||||||||
| 3. Be chemically and mechanically stable. | ||||||||||
| 4. Low electronic conductivity. | ||||||||||
| 5. Ease of manufacturability/availability. | ||||||||||
| 6. Preferably low cost. | ||||||||||
PHYSICAL DESCRIPTION OF PEM |
||||||||||
| The standard electrolyte material presently used in PEM fuel cell is a copolymer of poly tetra fluoro ethylene and poly sulfonyl fluoride vinyl ether. The polymer is stable in both oxidative and reductive environments and has high protonic conductivity (0.2S/cm). The thickness of these membranes ranges from 50 to 175 microns (mm). The protonconducting membrane usually consists of a PTFE-based polymer backbone, to which sulfonic acid groups are attached. The proton conducting membrane works well for fuel cell applications because the H+ jumps from SO3 site to SO3 site throughout the material. The H+ emerges on the other side of the membrane. This limits the operating temperature of PEM fuel cell. The SO3 sites in the Nafion membrane. Per fluoro sulfonic acid (PFSA) membranes, such as Nafion, have a low cell resistance for a 100-mm-thick membrane with a voltage loss of only 50 mV. | ||||||||||
| Figure 1 illustrates the chemical structure of Nafion. When modeling the polymer electrolyte membrane, it is typically assumed that the concentration of positive ions is fixed by electro neutrality, which means that a proton occupies every fixed SO3 − charge site. | ||||||||||
FUEL CELL MODELING |
||||||||||
| In order to model the electrolyte accurately, the transport of mass, charge and energy must be included in the model. Contact resistance between the electrode and the electrolyte can also be significant and should be incorporated into the model. | ||||||||||
| The equations and variables which are used to create a model for the polymer exchange membrane are given below. | ||||||||||
| A. Mass and Species Conservation | ||||||||||
| For both water and protons, the mass conservation equation can be represented as | ||||||||||
| where i is H2O, ci is the molar concentration, and Ni is the molar flux due to electro osmotic driving forces and convection. In a diluted solution, Ni is given by the Nernst-Planck equation along with the Nernst-Einstein relationship, | ||||||||||
| where um is the mixture velocity and Ji is the diffusive flux. | ||||||||||
| DcH2O, T is the diffusion coefficient, which includes a correction for the temperature and for the water content. It is expressed in a fixed coordinate system with the dry membrane by, | ||||||||||
| where a is the activity of water, and D’(m2/s) is the diffusion coefficient measured at constant temperature, and in coordinates moving with the swelling of the membrane. | ||||||||||
| The mass conservation of water can be expressed as, | ||||||||||
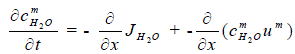 (4) (4) |
||||||||||
| where the mixture velocity um is given by the momentum equation. | ||||||||||
| Combining this diffusive flux with the convective flux results in the total molar flux for the hydrogen protons, | ||||||||||
| B. Momentum Equation | ||||||||||
| For the mixture of water and protons, the assumption is made that the momentum equation takes the form of the generalized Darcy relation as, | ||||||||||
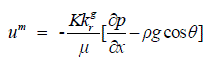 (6) (6) |
||||||||||
| where um is the mixture velocity, K is the absolute permeability of the porous medium, kg r is the relative permeability, g is the gravity, and θ is the angle that the x-axis (the direction of flow) makes to the direction of gravity. The mixture density and the dynamic viscosity of the mixture are written as, | ||||||||||
| C. Conservation of Energy Equation | ||||||||||
| Energy is transported by conduction and convection within the three phases of the membrane (polymer, liquid/gas). The effects of ohmic losses within the membrane are taken by an additional source term in the energy balance equation as given by, | ||||||||||
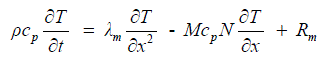 (8) (8) |
||||||||||
| The transient energy effect associated with mass storage within the hydrated membrane is neglected due to the fact that the dry membrane mass does not change, and is several orders of magnitude larger than that of the water that hydrates the membrane. | ||||||||||
| D. Ion Transport | ||||||||||
| The equation for the proton potential is derived from Ohm’s law, and represents the proton flux divided by the membrane conductivity. The electro neutrality assumption allows the total molar proton flux to be related directly to current density and the velocity um, represents the convective flux of protons which results in the following equation, | ||||||||||
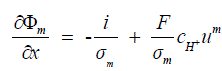 (9) (9) |
||||||||||
| Thus the fuel cell been modelled as a function using the acquired current and voltage measurement as given in equation (10), | ||||||||||
CONTROLLER DESIGN AND RESULTS |
||||||||||
| There are many different type of control strategies developed to improve the performance of the fuel cell system. For example, a Fuzzy control strategy is developed in [3] to improve the performance of the fuel cell system which is based on system error and its rate of variations. In [2] an online self tuning PID controller for PEM fuel cell systems is described. However, from the literature it can be seen that the PID controller design is widely used for performance enhancement in fuel cell systems. A proportional-integral-derivative controller is a generic control loop feedback mechanism (controller) which is widely used in industrial control systems. As the study of PEM fuel cell proved better performance when comparing to the hydrogen fuel cell related to the optimized output. The figure below shows the simulation result of PEM fuel cell. As the study of PEM fuel cell proved better performance when comparing to the hydrogen fuel cell related to the optimized output. | ||||||||||
| During the first 10 secs, the utilization of the hydrogen is constant to the nominal value H2 = 99.56% using a fuel flow rate regulator. After 10 secs, the flow rate regulator is bypassed and the rate of fuel is increased to the maximum value of 85lpm in order to observe the variation in the voltage. The voltage and current waveforms of PEM fuel cell is as shown above. It is observed that from the simulation results the variations in the input maintains the output current constant. | ||||||||||
CONCLUSION |
||||||||||
| The model of Proton Exchange Membrane (PEM) fuel cell was simulated using MATLAB. Inputs are given to the proposed fuel cell system to achieve the desired output voltage and output current. The results show that the fuel cell system improves the stability and performance of the system. Thus the results prove that the design of proposed controller is validated. Future work is now proceeding to expand this strategy to investigate the hybrid modeling approach. This will be followed by extending the work to include an additional optimization algorithm to adjustable parameters in the model be more accurate. | ||||||||||
Figures at a glance |
||||||||||
|
||||||||||
References |
||||||||||
|