The polycrystalline Hg- doped ZnS thin films semiconductor photoelectrodes of various compositions were prepared by chemical bath deposition technique. The deposition conditions and preparation parameters are optimized to have good quality of films. As-deposited films were made use as an active photoelectrodes in a photoelectrochemicalcellwithpolysulfide (1M) as an electrolyte and impregnatedgraphite as the counter electrode. As fabricated photoelectrochemical (PEC) cells are then used to study their optical characteristics. The spectral response characteristics of the films at room temperature were studied under the constant illumination of light of intensity 20 mWcm-2. Mott-Schottky plots were also studied and the flat band potential (Vfb) is found to be increased with electrode composition x and reached to its maximum value at x = 0.1. Transient response times of as-deposited photoelectrodes of different compositions were studied.
Keywords |
| pseudo-binary MZS, CBD, PEC cells, flat band. |
INTRODUCTION |
| A binary and ternary compounds of II-VI, IV-VI or II-IV-VI are most dominant semiconductor groups in thin films
for photo-voltaic and detector configurations Many researchers have been working on the development of
semiconductor materials by forming solid solutions of different compositions to obtain variation in flat band potential
and optical energy gap of alloyed materials, which capture a large fraction of the ultraviolet, visible and near infrared
regions of the electromagnetic spectrum and for the electrolytes, which are suitable for better stability.Enhancementin
photo-potential and photo-current have been observed insuch systems which are being attributed to the shift in flat band
potential which increasesthe barrier height at a junction. |
| Chalcogenides, in general because of their potential as efficient absorbers in the visible and near IR regions of solar
radiations, have attracted more and more researchers in the field of detection and photoelectrochemical conversion.
These materials show a good deal of a wide range of photon energy Low fabrication cost, ease in operationand
relatively no lattice mismatch, are the great advantages of PEC cells over the thin films solid solar cells have betterand
efficient solar energy conversion devices [1] -[5]. |
| To understand the importance of solid state parameter we deposited the Hgx Zn1-xS thin films by inexpensive, simple
and easy to operate chemical bath deposition method. The photoelectrochemical performance of these cells has been
studied and presented in this paper. |
EXPERIMENTAL PROCEDURE |
A. Preparation of thin film electrodes |
| Hgx Zn1-xS (0 ≤ x ≤ 1) thin films were deposited ontothoroughly cleanedglass substrate(Blue Star, Mumbai) and high
quality stainless steel substrate using chemical bath deposition method. For this purpose, HgCl2 (1M), ZnSO4.7H2O
(1M) and CS (NH2)2 in stoichiometric proportions were taken in a reaction vessel. Triethanolamine was added to form
a bound complex. Sodium hydroxide and aqueous ammonia were added to this reaction mixture to adjust the pH of the
reaction and to increase the film adherence to the substrates.The deposition parameters viz.temperature, speed of
mechanical churning, time of deposition, pH etc.was kept constant at their optimized values of 60oC, 60 ± 2 rpm, 90
minutes and 10±0.2 as reported earlier[6] -[7]. |
B. Fabrication of Hgx Zn1-x S photoelectrochemical cells |
| The photoelectrochemical (PEC) cells were fabricated for different electrode material compositions, as an active
photoelectrode and polysulfide as a suitable redox couple in an H-shaped corning glass corvette set. An impregnated
graphite rod acts as a counter electrode which is placed behind the active photoelectrode at a distance of 2mm. The
current–voltage characteristics in dark and under illumination were studied and examined at room temperature. The cells are illuminated by the light of 20 mW/cm2intensity and photo-voltaic power output curves were obtained for all
these cells.A potentiomateric arrangement was used for measurement of current – voltage capacitor-voltage at various
applied bias potentials for dark conditions.The spectral response of the various cells was examined in the range of
wavelength from 350 to 950 nm. Digital picometer of 3
1
2
digits, SES Instrumentation Pvt. Ltd., classic (999N1) digital
Multimeter and Aplab’sAutocoupler LCR-Q meter were used for the measurement of current, voltage and capacitor
respectively. |
RESULTSAND DISSCUSSION |
A. Physical properties |
| HgxZn1-xS thin films of various compositions are deposited onto the well-polished stainless steel substrate at by
chemical bath deposition process. For this purpose, the preoperative parameters such as temperature, deposition time,
pH, and speed of the substrate of rotation were kept constant at their optimized value.The as-grown films are thin,
uniform, mechanically hard and adherent to the substrate support. It is seen that the layer thickness decreased
monotonically with composition parameter. The thin film properties of these films viz. structural, optical and electrical
transport properties have studied and reported earlier [6]-[7]. |
B. Photoelectrochemical (PEC) Studies |
| The photoelectrochemical cell was prepared by using thin films of HgxZn1-xS as an active photoelectrode for current –
voltage, capacitance-voltage, power output and optical characterization. The cell performance, junction idealitywas
determined from forward and reverse leakage I-V characteristics under the dark condition. It is seen that considerable
recombination at the electrode/electrolyte interface and its values are cited in the table. In order to determine the flat
band potentials for different cell, Mott-Schottky plots were constructed and the flat band potentials were determined by
extrapolating the steep portion of the curves on voltage –axis and its values are cited in the table. It is observed that the
flat band potential was maximum at composition x= 0.1 and then decreased as composition x increases. The increase in flat
band potential is due to increase in surface deposition and formation of new donor levels in the band gap of the
semiconductor electrode, Which shift the Fermi level in an upward direction causing an increase in build in potential of
the cell. The generation of scattering centers in the electrode material,is as a result of excess incorporation of Hg
content and surface states at the interfacing of electrode and electrolyte.[3],[8]-[11] |
C. Spectral Response |
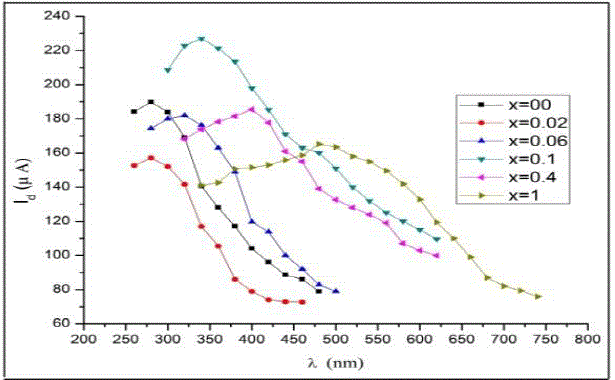
| To study the spectral response of the material the PEC cells were illuminated by incident light at constant intensity
(20mW/cm-2) for the different wavelength from 250 to 800 nm. The corresponding photocurrents weremeasured as shown in the fig. 1.The graph shows that the photocurrent increased with incident wavelength and maximum at the cutoff
frequency (λc) equals to 348.69nm and 626.46 nm for cells of composition x = 0 and x = 0.1 respectively. There
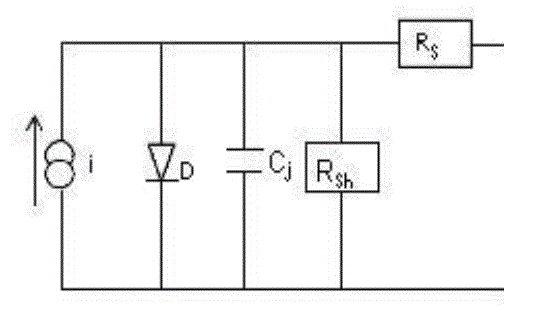
after photocurrent decreased for higher wavelengths. The longer wavelength cut-off is not sharp which is in accordance
with its theoretical model of the low pass filter circuit as shown in the fig. 2 [3],[12]-[15]. |
| The cut-off frequency (fc) and response time (τ) of this low-pass filter is given by, |
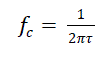 |
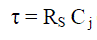 |
D. Photoresponse |
| To study the photoresponsivity of as-deposited electrodes for different composition, electrodes were illuminated by
light of wavelength (λc = 810 nm). Photo-potential (Vph) and photocurrent (Iph) were recorded by varying intensity of
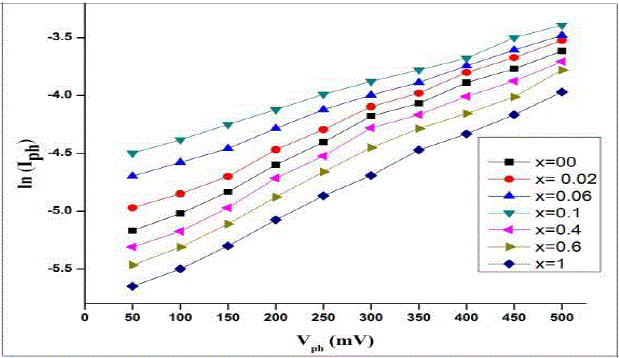
incident light from 5 to 20 mWatts/cm2. The photo-response of these cells were studied by constructing plots of ln (Iph)
vsVph as shown in fig. 3 to calculate the lighted quality factor (nL) of the junction and their values are cited in the table.From the fig. 3 it is observed that (Vph) and (Iph)increased with
the composition x andreached to its maximum value at x = 0.1. Thisbehaviour of electrodes is mainly due to increase in
the flat band potential and decrease in electric resistivity, an optical energy gap of the photoelectrochemical cells. [13]-
[14]. |
E. Transient Time |
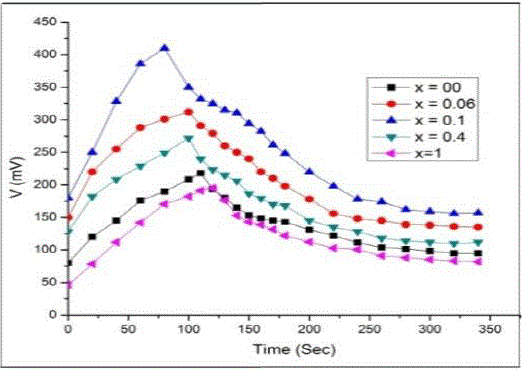
| To understand the photo response of cells, the electrodes of different composition were illuminated by light of suitable
wavelength. The photo-potentials were measured as a function of ON and OFF time for all these cells. Fig. 4 shows the
variation of photo potential with ON and OFF times for few selected configurations. From these plots, it is clear that
the transient response is exponential in nature, when the light source is ON photo potential increases exponentially with
time, this is a clear indication of something analogous to the charging of a capacitor when connected across the battery.
Similarly when light source is cut (OFF), the decrease in photo potential behaves as if the discharging of a capacitor. |
| Thus the three main factors which limit the switching speed (response time) of photo electrodes in
photoelectrochemicalcells,are as follows [12]-[13]. |
| Diffusion time of photo carriers to the depletion region. Carrier diffusion is inherently a slow process; the time
taken for carrier to diffuse is given byπdiff=
d2/2
dc
where (d) distance and Dc minority carrier diffusion
coefficient. At wavelengths near the band gap and at low reverse bias speed of some optically generated
carrierslimit the diffusion. The carriers generated within the depletion region responses rapidly, whichgives to
slow decrease of photo potential. |
| 2. Drift time of carriers through the depletion region. The carries which are generated near to the one edge of
deletion region has to spend longest transit time, so it can travel full depletion region. |
| 3. Junction capacitor effect. The bandwidth may improve by reducing junction capacitorand it can be done by
reducing area (A) and doping level (Nd) or increasing the reveres bias voltage. This leads to increases in the
depletion width and the drift transit time. |
CONCLUSION |
| Thin films of Hgx Zn1-x S of various composition x were deposited by chemical bath deposition. As deposited
electrodes of different composition are in conjunction with polysulfide electrolyte can be used as photovoltaic cell.
Spectral response study of shows electrochemical cells are of analogous to a low pass filter. The response time of a cell
shows similarnature to its solid –solid junction counterpartand response time depends on electrodes composition and it
is optimized at x= 0.1. |
ACKNOWLEDGEMENTS |
| One of the authors (ARP) acknowledges with thanks to the Principal of KBP college, Vashiand Bhavan’s college
Andheri. He also thanks the staff of the Bhavan’s college for their support and encouragement. |
Tables at a glance |
 |
| Table 1 |
|
Figures at a glance |
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
|
References |
- Miller B & Cleveland J. R., ‘Semiconductor liquid junction solar cells based on anodic sulphide films’, vol. 262,pp680(1980).
- D. R. Kendre, A. R. Pawar, V. B. Pujari, ‘Optical and microscopic studies of Pb Doped DdSeThin films’ PDFARDIJ, Vol, No 6, Issue no 14, pp125-131,(2012).
- V. B. Pujari, D. J. Dhage and L. P. Deshmukh, ‘n-HgxCd1-x Se thin film electrodes for photoelectrochemicalapplications’ ,Ind.J.ofEngg. andMater.Sciences, Vol. 15, pp 275-280, (2008).
- E. U. Masumdar, L. P. Deshmukh, S.H. Mane, V. S. Karande, V. B. Pujari and P. N. Bhosale, ‘CdSe:Sb electrode for photoelectrochemical application’, J. of Mater. Sci.: Mater.in Elect.’, Vol. 14, pp 43-48, (2003).
- V. B. Patil and L. P. Deshmukh, ‘Photoelectrochemical investigation on n-CdS1-xTex thin film electrode/polysulphide system’,Int.J.Electronics, Vol. 91 pp 13-23, (2004).
- Ashok R. Pawar, Dnyanoba R. Kendre and V. B. Pujari, ‘Electrical transport and Spectral Response Hg Zn S thin films’, Internat.J. of Adv. in Elect. And Electron. Engg, Vol .2, iss.1 pp 68-73(2012).
- Ashok R Pawar , Dbyanoba R. Kendre and V. B. Pujari, ‘Synthesis, Growth and Compositional Studies of Mercury doped zinc Sulfide Thin Films’, PDFARDIJ (Print) Vol.6,Iss.6 pp 58-65, (2012).
- Ashok R.Pawar ,DyanobaR. Kendre and V. B. Pujari,‘ Photo-voltaic Study of Hg Doped ZnSThin Films’, IJAIEM,Vol :2, issue 2,pp285-289, (2013).
- M. A. Barote, A.A. Yadav, T. V. Chavanand E. U. Masumdar, ‘Characterization and PhotoelectrochemicalPropreties of Chemical BathDeposited n-PbS Thin Films’, Digest j. of nanometer. AndBiostruct., Vol. 63 pp 979-990,(2011).
- V.B. Pujari and L. P. Deshmukh, ‘Photoelectrochemical Investigation on HgxCd1-x Se Thin Film Electrode/ Electrolyte System’, Turk.J.Phys.,vol. 32, pp 1-10, (2008).
- V. S. Karande, S. H. Mane, V. B. Pujari, L.P. Deshmukh, ‘ Elctrochemical studies of n-Cd1-x Mnx Se thin film photodetector’, J. of Mater. Sci.: Mater.in Elect.’, Vol. 15, pp 419-423, (2004).
- Tiwari J. N. ‘Solar energy fundamental, design, modelling and application ‘Narosa publication, New Delhi, (2002).
- Wilson J., Hawkes J.F.B., ‘Optoelectronics; An Introduction’, Prentice- Hall Of India, New Delhi, (2001).
- Keiser G, ‘Optical fiber communication ‘McGraw-Hill Inc. New York, (1991).
- S. J. Lade and M. D. Uplane and C.D.Lokhande, ‘Photoelctrochemical properties of CdX( S=S,Se,Te) films electrodeposited from aqueous and non-aqueous bath’, Mater. Chem.Phys., 68, pp-36-41, (2001).
|