ISSN:2321-6212
ISSN:2321-6212
Ravi Shrivastava1*, Ishwar Prasad Sahu2
1 Department of Science and Technology, The ICFAI University Raipur, Raipur, India
2 Department of Physics, Indira Gandhi National Tribal University, Amarkantak, India
Received: 12-Jan-2022, Manuscript No. JOMS-22-51610; Editor assigned: 14- Jan -2022, PreQC No. JOMS -22-51610(PQ); Reviewed: 26- Jan -2022, QC No. JOMS -22-51610; Revised: 28- Jan -2022, Manuscript No. JOMS -22-51610(R); Published: 04-Feb-2022, DOI: 10.4172/2321-6212.10.2.003.
Visit for more related articles at Research & Reviews: Journal of Material Sciences
Di Barium Magnesium Silicate (Ba2MgSi2O7) doped with various concentrations (0.2, 0.5, 1.0, 2.0 and 2.5 mol %) of Europium (Eu3+) were prepared using solid-state reaction technique. To confirm the proper preparation and the phases available, an X-ray diffraction pattern of the sample with optimal PL concentration was recorded. The recorded sample was matched significantly with crystallographic open database card No. 96- 900-6941 reported specifically for Ca2MgSi2O7 with a Figure of Merit (FoM) of 0.8051. This FoM confirmed the proper preparation of the sample. Rietveld lattice parameter refinement process using Celref V.2 was carried out for estimating the various crystallographic information of the prepared sample. The prepared samples underwent Photoluminescence (PL) studies. The excitation spectrum, which was monitored at 620 nm, exhibited two distinct peaks centred at 294 nm and 365 nm respectively. Therefore, the emission spectra were recorded at an excitation wavelength of 294 nm. The emission spectra expressed the prominent peaks centred at 592 and 612 nm are attributed to 5D0 →7F2 of Europium (III) ions accommodated at various lattice sites having different energies, whereas peak centred at 633 nm is due to 5D0 →7F3 transition of Europium (III). Sample with a dopant concentration of 2 mol % expressed the maximum PL intensity. Overall emission was found in the red colour region which was confirmed using a CIE chromaticity diagram with coordinate (0.611, 0.387). Critical distance for energy transfer in the concentration, beyond which concentration quenching occurred in PL spectra, was calculated. In this case, the critical distance was found to be 19.34 Å, therefore the mechanism involved in concentration quenching of Ba2MgSi2O7 doped with 2.0 mol % of Europium (III) must be only multipole-multipole exchange whereas the exchange interaction is ineffective.
Red LED; Phosphor; Photoluminescence; Rietveld refinement; XRD; Europium
Europium activated di alkaline earth (Sr, Ba, Ca) magnesium silicate materials have been investigated extensively because of their excellent luminescence properties like broaden emission peak, long lasting behavior and ability to retain these properties even inside the liquids. Silicate based phosphor has usually a band gap around 7.5 eV, which make these phosphors suitable for Light Emitting Diodes (LEDs) and Plasma Display Panel (PDPs) [1-4]. White LEDs have been produced commercially using a combination of two colour phosphor i.e. blue and yellow colour. In this case fabrication cost is low and luminescence efficiency is quite high but the colour rendering is poor [5-6]. To get rid of this problem, one of the options was to compensate this deficiency of the red element of Yttrium Aluminum Garnet (YAG) doped with Ce3+ phosphor-based LED with another red-emitting phosphor separately[7-8]. Unfortunately, the phosphors studies were limited to low brightness and could not absorb efficiently in the near UV region, even the lifetime was also not inadequate under near UV irradiation. Tri colour phosphor with strong green, yellow and red luminescence is suitable for high colour rendering white LEDs. Most of the green emitting phosphors for White LED application are nitride phosphors. "The red colour emission of Eu3+ is a result of the intra-4f transitions, while the emission of Eu3+ from the 5d–4f transition which is dipole allowed varies in a significantly large range from red to UV region which depends in accordance to the crystal structure of the host materials. It is well known that the optical properties of rare-earth ion-doped luminescent materials are highly affected by the matrix[9]. Therefore environment-friendly and stable red-emitting rare-earth-doped silicate-based phosphors have attracted many researchers. In this article, Ba2MgSi2O7 doped with various concentrations of Eu3+ were prepared and its photoluminescence studies were done.
Eu3+ doped Ba2MgSi2O7 phosphors doped with different concentration of Eu3+ (0.5, 1.0, 1.5, 2.0 mol %) were prepared using solid state reaction technique. The starting materials used were SiO2, BaCO3, MgO, and Eu2O3 with proper stoichiometric proportions were thoroughly ground for approximately 1 h in a mortar, pre-sintered at 900oC, then fired at 1200oC for approximately 2 h, with 1.6 mol% of H3BO3 used as flux[10-13]. The Photoluminescence (PL) excitation and emission spectra were measured by a spectrofluorophotometer (SHIMADZU, RF-5301 PC, Kyoto, Japan) using a Xenon lamp of power 150 watt as excitation source. X-Ray Diffraction (XRD) characterization of the sample is done using Panalytical Xpert PRO MPD (Singapore) with copper k alpha anode of wavelength 1.5405 AÃâ¹ÃÅ¡.
XRD analysis:
Figure 1 exhibits the comparison of observed and calculated XRD pattern of Ba2MgSi2O7 having 0.5 mol% of Europium (III). The results show a good resemblance between the two graphs. Match! v. 2.3.1 was used to match the standard (Crystallographic open database) and experimental XRD pattern. It was observed that, experimental XRD pattern was matched convincingly with Crystallographic Open Database (COD) card No. 96-900-6941 with Figure of Merit (FoM) of 0.8051. This is already reported XRD pattern of Ca2MgSi2O7.
The lattice parameter refinement process was carried out using software Celref v.2. The crystallographic information file (1010215.cif) corresponding to the Crystallographic Open Database COD 96-900-6941 was downloaded from the website http://www.crystallography.net/. The information contained in the crystallographic information file was utilized as reference in the process of lattice parameter refinement. Initial and refined lattice parameters are shown in the Table 1.
| Zero | Lambda | a | b | c | alpha | beta | gamma |
|---|---|---|---|---|---|---|---|
| 0 | 1.5418 | 7.73 | 7.73 | 5.01 | 90 | 90 | 90 |
| 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| Final values: (Standard errors on 2nd line) | |||||||
| Zero | Lambda | a | b | c | alpha | beta | gamma |
| 0 | 1.5418 | 7.6306 | 7.6306 | 5.2039 | 90 | 90 | 90 |
| 0 | 0 | 0.1259 | 0 | 0.1325 | 0 | 0 | 0 |
| H | K | L | 2T(Obs) | 2T-Zero | 2T(Cal) | Diff | |
| 1 | 2 | 0 | 25.601 | 25.601 | 26.1123 | -0.5113 | |
| 1 | 2 | 1 | 31.175 | 31.175 | 31.3458 | -0.1708 | |
| 2 | 2 | 0 | 32.926 | 32.926 | 33.2074 | -0.2814 | |
| 2 | 2 | 0 | 35.178 | 35.178 | 33.2074 | 1.9706 | |
| 2 | 2 | 1 | 37.494 | 37.494 | 37.5517 | -0.0577 | |
| 2 | 1 | 2 | 44.141 | 44.141 | 43.7491 | 0.3919 | |
| 0 | 4 | 0 | 46.792 | 46.792 | 47.671 | -0.879 | |
| 3 | 3 | 1 | 53.72 | 53.72 | 53.9373 | -0.2173 | |
| 5 | 1 | 2 | 72.795 | 72.795 | 72.9207 | -0.126 | |
Table 1. Initial and refined lattice parameter of Ba2MgSi2O7 doped with Eu3+.
Photoluminescence studies
Photoluminescence Excitation spectrum (Figure 2) of the Ba2MgSi2O7 doped with 1.5 mol% Europium (III) was monitored at 592 nm. Observed spectrum exhibited two peaks centered at 294 and 364 nm respectively, therefore the emission spectra of the samples with various concentration of Europium (III) i.e. 0.2, 0.5, 1.0, 2.0, and 2.5 mol% were recorded for an excitation wavelength of 294 nm. The emission spectra which were monitored by giving an excitation wavelength 294 nm are expressed in Figure 3.
The emission spectra expressed the prominent peaks centred at 592 and 612 nm are attributed to 5D0 →7F2 of Europium (III) ions accommodated at various lattice sites having different energies, whereas peak centred at 633 nm is due to 5D0 →7F3 transition of Europium (III) [14]. Sample with a dopant concentration of 2 mol % expressed the maximum PL intensity. Overall emission was found in the red colour region which was confirmed using a CIE chromaticity diagram (CIE 1931) with coordinate (0.4805, 0.3763) (Figure 4) which is the point belonging to the intense red colour region.
Figure 3 expressed the variation in peak emission intensity for various Eu3+ doping concentrations. We noticed from Figure 4 that the emission intensity increases with increasing concentration of Eu3+ ion till it reaches 2.0 mol% and then decreases for 2.5 mol% doping of Eu3+. This is dueF to concentration quenching. When the concentration of Eu3+ ions increases, the distance between the Eu3+ ions decreases. This results in the non-radiative transitions in Eu3+ ions. It points an important fact that the transfer of excitation energy strongly depends on the distance between the Eu3+ ions. Blasse [13,15] gave an important formula to estimate the critical distance for energy transfer (R).
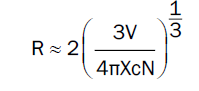
Where V is the unit cell – volume, N is the number of atoms per unit cell, and Xc is the critical concentration of the dopant. In present case (Ba2MgSi2O7 doped with Eu3+ - 2.0 mol %) the unit cell volume is 303.0 (Å)3, Number of atoms per unit cell is 4 and the critical concentration is 0.020. Using the Blasse’s formula, the value of critical distance was found as 19.34 Å. The non-radiative energy transfer of the luminescence of oxidic phosphors is based the resonance transfer by electric multipole -multipole interaction or exchange interaction[16].
This situation was explained by the Blasse’s theorem [13,17]. It was reported that exceeding critical distance beyond 5 Å, only a multipole-multipole interaction is important where the exchange interaction becomes ineffective. When the critical distance is found less than 5 Å then, the exchange interaction becomes effective [18]. In present case the critical distance is calculated as 19.34 Å therefore the mechanism involved in concentration quenching of Ba2MgSi2O7 doped with 2.0 mol % of Eu3+ is considered to be the multipolar interaction.
Di Barium Magnesium Silicate (Ba2MgSi2O7 ) doped with various concentrations (0.2, 0.5, 1.0, 2.0 and 2.5 mol %) of Europium (Eu3+) were prepared using solid-state reaction technique. The sample with optimum PL emission was characterized by means of X-ray diffraction pattern, which was well matched with the standard reported for the similar sample. The PL excitation spectrum, which was monitored at 620 nm, exhibited two distinct peaks centred at 294 nm and 365 nm respectively. Therefore, the emission spectra were recorded at an excitation wavelength of 294 nm. The emission spectra expressed the prominent peaks centred at 592 and 612 nm are attributed to 5D0→7F2 of Europium (III) ions accommodated at various lattice sites having different energies, whereas peak centred at 633 nm is due to 5D0 →7F3 transition of Europium (III). Sample with a dopant concentration of 2 mol % expressed the maximum PL intensity. Overall emission was found in the red colour region which was confirmed using a CIE chromaticity diagram with coordinate (0.611, 0.387). The CIE coordinate was in the region of intense red colour region. As per the literature this intense red colour emission can be a potential candidate for being red colour Light Emitting Diodes. Critical distance for energy transfer in the concentration, beyond which concentration quenching occurred in PL spectra, was calculated which indicated that there must be only multipole-multipole exchange whereas the exchange interaction is ineffective.
[Cross Ref] [Google scholar] [Pubmed]
[Cross Ref] [Google scholar] [Pubmed]
[Cross Ref] [Google scholar] [Pubmed]