ISSN: 2320-0189
ISSN: 2320-0189
Changhong Yao1*, Yadong Chu2, Yinghui Liu2 and Xupeng Cao2
1Department of Pharmaceutical and Biological Engineering, School of Chemical Engineering, Sichuan University, Chengdu, P.R. China
2Marine Bioengineering Group, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, P.R. China
Received Date: 16/12/2017 Accepted Date: 19/12/2017 Published Date: 27/12/2017
Visit for more related articles at Research & Reviews: Journal of Botanical Sciences
Microalgal starch is considered to be an alternative to the crop-based starch with variety of applications. Nitrogen limitation is an effective method to trigger starch accumulation in microalgae. The relationship between starch accumulation and photosynthetic performance of microalgae have to be disclosed to balance the inconsistency between starch accumulation and lose of photosynthetic activity under nitrogen limitation. This study aimed to investigate the photosynthetic performance in relation to nitrogen limitation-induced starch production in a marine green microalga Tetraselmis subcordiformis. The highest starch productivity of of 0.50 g L-1 d-1 and starch content of 60.5% DW were both obtained in the culture with medium initial nitrate concentration of 1.0 mM on Day 2. Along with the moderate decline of maximum quantum efficiency of photosystem II (FvFm) and photosystem II operating efficiency (ΦPSΙΙ) therein, it was evidenced that moderate stress condition was preferable for maximal starch production. Much less increase of Chl a/b ratio, an indicator of decreased antenna size, was found in the culture with less initial nitrate and more starch accumulation, suggesting that photoinhibition should be inherently a prerequisite for starch production in T. subcordiformis. The more prominent increase of non-photochemical quenching (NPQ) was accompanied by the stronger starch accumulation ability, indicating that the maintenance of photoprotective function via heat dissipation and Cyclic Electron Flow (CEF) around photosystem I (PS I) could also be of importance for the starch accumulation in T. subcordiformis.
Microalgal starch, Tetraselmis subcordiformis, photosynthesis, Nitrogen limitation, FvFm, ΦPSΙΙ, Chl a/b ratio, Nonphotochemical quenching.
Microalgae are photosynthetic microorganisms that absorb light as their energy and fix CO2 along with other inorganic nutrients (e.g. nitrate, phosphate, sulfate, etc.) for growth and proliferation [1]. Microalgae have been regarded as potential feedstock for bioenergy and high-value compounds production due to its high photosynthetic efficiency relative to higher plants, fast growth, robust CO2 fixation ability, readily adaption to extreme environments, and no competition for arable lands [2]. Starch serves as the primary photosynthetic carbon reserve in many species of microalgae [3,4]. Due to their similar structures with crop-based starch such as rice, maize, wheat, corn, and potato, microalgal starch is considered to be an alternative source of food, industrial raw materials, and renewable energy such as bioethanol, biobutanol, biohydrogen, and biogas [5-7].
The accumulation of starch in microalgae was largely dependent on the environment the microalgae have been exposed to. Under unfavorable conditions such as high light exposure, low nutrient availability, and aberrant in isotonicity, microalgae tend to synthesize and accumulate starch in cells [3,8-10]. Among these environmental factors that trigger starch accumulation, nitrogen limitation is the most commonly acknowledged one. It is believed that nitrogen limitation tends to divert the carbon flux from the biosynthesis of nitrogen-containing compounds (e.g. protein, nucleic acid, and chlorophyll) towards the nitrogen-free molecules such as starch and oils [11,12]. In the model microalga Chlamydomonas reinhardtii, nitrogen starvation led to 10-fold increase in starch content in cells [13], and the starch content could reach to 35%-58% [14,15]. Similar results had been recorded in other green microalgae like Chlorella sp. [16,17], Scenedesmus obliquus CNW-N [18], and Chlorococcum littorale [19], and some cyanobacteria like Arthrospira platensis [20], Synechococcus sp. 7002 [21], and Synechocystis sp. PCC6803 [22]. Although nitrogen limitation is an ef- fective way to induce starch accumulation in microalgae, there is a significant challenge as to how to enhance starch production without diminishing biomass accumulation since nitrogen limitation inevitably reduces photosynthetic activity [23]. Therefore, it is vital to balance the inconsistency between starch accumulation and lose of photosynthetic activity under nitrogen limitation. To this end, the relationship between starch accumulation and photosynthetic performance of the microalgae have to be dissected.
Tetraselmis subcordiformis is a marine green microalga that have been demonstrated to possess excellent ability of producing starch under nutrient limitation and low salinity conditions [8-10]. Previous study had revealed a relationship between starch production and stress levels the microalgae were subjected to which was represented by a chlorophyll fluorescent dynamics parameter, maximum quantum efficiency of photosystem II (FvFm) [8]. In this study, more chlorophyll fluorescent dynamics parameters, e.g. photosystem II operating efficiency (ΦPSΙΙ), and non-photochemical quenching (NPQ) were applied to make a clearer picture of photosynthetic performance in relation to nitrogen limitation-induced starch production in this microalga.
Strains and Culture Conditions
Tetraselmis subcordiformis FACHB-1751 was isolated from the Huanghai Sea near Dalian, Liaoning Province, P. R. China and maintained by the Freshwater Algae Culture Collection of the Institute of Hydrobiology (FACHB collection), Chinese Academy of Sciences. The microalgae were previously cultivated in natural seawater by adding nutrients, as described by Yao [8]. Algal cells were harvested during the late exponential phase and washed twice with nitrogen-free artificial seawater (ASW-N) according to Yao [9]. The washed cells were resuspended in ASW-N before nitrate was added to meet the desired nitrate concentrations of 0.5 mM, 1.0 mM, and 2.0 mM.
The cells were cultivated in a 600-mL glass air bubble column photobioreactor (50 mm diameter, 400 mm height) with a working volume of 500 mL and an aeration of 0.4 vvm with air containing 3% CO2 at 25±2°C under an incident light intensity of 200 μmol m–2 s–1 as described by Yao [8]. Each nitrate condition was set for at least two biological replicates.
Growth and Biochemical Composition Measurement
The cell dry weight (DW), starch and chlorophyll (Chl) were determined according to Yao [8]. Starch productivity (Ps, g L–1 d–1) was calculated as follows:
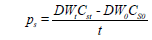
where Cst and Cs0 are the starch content at culture times t and 0, respectively.
Chlorophyll Fluorescence Measurement
The chlorophyll fluorescence was measured using a chlorophyll fluorometer (Water-PAM WALZ, Germany) as described previously [9,24]. The maximum quantum efficiency of photosystem II (FvFm), photosystem II operating efficiency (ΦPSΙΙ) and Non-Photochemical Quenching (NPQ) were calculated according to Baker [25]:
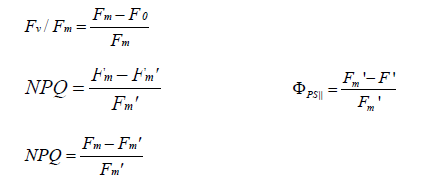
Starch Production under Nitrogen Limited Conditions
Previous study showed that nitrogen limitation could occur within one day with an initial nitrate concentration down to 3.0 mM [8]. To further aggravate the nitrogen stress and enhance starch production, a series of initial nitrogen concentrations, from 0.5 mM to 2.0 mM, were applied herein.
As shown in Figure 1a, the cell growth was positively related to initial nitrate concentration in the medium. The biomass concentration increased by 2.6 times within three days in the cultivation with an initial nitrate concentration of 2.0 mM, whereas the one with 0.5 mM nitrate increased by only 1.6 times. The starch content in all the cultivations increased from the beginning of the cultivation and peaked on the second day, after which the starch levels declined (Figure 1b). Noteworthily, the starch accumula tion in the culture with medium initial nitrate (1.0 mM) significantly exceeded that with 2.0 mM or 0.5 mM of initial nitrate, with the highest starch content of 60.5% DW obtained on the second day. The highest starch productivity, 0.50 g L-1 d-1, was also achieved in the culture with medium initial nitrate (1.0 mM) on Day 2 (Figure 1c). The starch concentration in the cultures with 0.5 mM and 1.0 mM of initial nitrate increased in the first two days while remained unchanged or even decreased on the third day (Figure 1d). In contrast, higher initial nitrate (2.0 mM) led to consecutive starch production up to Day 3, though the starch productivity also decreased from Day 2 (Figure 1c). Collectively, these results demonstrated that an optimal initial nitrate concentration of 1.0 mM was present in the cultivation of T. subcordiformis for starch production in regard to the achievement of best starch content and productivity. Similar results were found in a cyanobacterium Arthrospira platensis that 3 mM of initial nitrate was preferable for optimized glycogen production in terms of glycogen content and productivity [20].
Figure 2 summarized the starch productivity and starch content on the second cultivation day in T. subcordiformis under different initial nitrate conditions conducted in the same cultivation system both in our previous (0, 3.0, 6.0, and 11.0 mM) [8] and present (0.5, 1.0, and 2.0 mM) studies. It was evident that the maximum starch productivity of 0.50 g L-1 d-1 and starch content of 60.5% DW were both achieved under the culture with 1.0 mM of initial nitrate. This starch production ability in T. subcordiformis was superior to most of the microalgae under nitrogen limitation reported hitherto except for some freshwater microalgae such as Chlorella who could reach a starch productivity up to 0.730 g L-1 d-1 within two days yet the light intensity was 4-fold higher than that in the present study [16].
Photosynthesis is essentially the original driver for starch production in microalgae. In order to dissect the underlying key photosynthetic factors regarding the starch accumulation, the chlorophyll content variations and photosystem II chlorophyll fluorescence dynamics were tracked during the cultivation. Under nitrogen limitation, the chlorophylls (Chl), including both Chl a and Chl b, declined drastically in all the cultures tested (Table 1). The decrease in Chl was physically due to the shortage of nitrogen since Chl molecules contain large amount of nitrogen. More importantly, this decline was a universal strategy for algae to protect themselves from photodamage by reducing the photons absorbed by the pigments when algae were subjected to stress conditions [12]. It was evident from Table 1 that the Chls declined more rapidly in the cultures with less initial nitrate available. For example, the total Chl (a+b) decreased by 62% in the culture with initial nitrate concentration of 0.5 mM, while in the 1.0 mM and 2.0 mM counterparts the decline levels reached 61% and 55%, respectively. These results complied well with the variations of photosynthetic activity represented as maximum quantum efficiency of photosystem II (FvFm) and photosystem II operating efficiency (ΦPSΙΙ) (Figures 3a-3c): the less initial nitrate was available, the more rapid decline of FvFm and ΦPSΙΙ were observed. Fv/ Fm is considered to be a sensitive indicator for stress conditions: the decline of FvFm represents a stress environment that the microalgae have been exposed to [26]. ΦPSΙΙ usually displays a similar trend with FvFm since the photosystem II operating efficiency is closely related to stress conditions [25]. Collectively, it was indicated that less nitrogen led to more severe stress and hence resulted in more damage on photosynthesis. As discussed above, the optimal starch accumulation occurred in the culture with medium initial nitrate available (1.0 mM), not the ones with higher or lower initial nitrate levels, suggesting that moderate stress condition was preferable for maximal starch production. This result agreed with our previous findings that adequate photosynthetic activity was required for starch accumulation [8].
Interestingly, nitrogen limitation led to an increased Chl a/b ratio, and this phenomenon was much more prominent in the culture with 2.0 mM of initial nitrate (Figure 3a). Table 1 revealed that this could be accounted for the more rapid decline of Chl b content relative to Chl a, especially in the 2.0 mM culture. For instance, both Chl a and Chl b declined to 45% of the original level in the 1.0 mM culture on Day 2, whereas Chl b declined to 55% but Chl a to 57% in the 2.0 mM culture. The increase of Chl a/b ratio under nitrogen limitation was also documented in other microalgae such as Chlorella sorokiniana and Scenedesmus sp. [27,28]. This was believed to be an indication of decreased light harvesting complex or antenna size [29]. It could serve as a protective function against oxidative stress resulted from nitrogen shortage in the presence of excessive light energy [28]. It is acknowledged that protein synthesis in microalgae is immediately suppressed upon nitrogen shortage, which mostly hinders the protein turnovers of photosynthetic apparatus especially photosystem (PS) II D1 reaction center protein [30]. This will lead to a declined photosynthetic electron transport rate (ETR) and consequently a reduction in photochemical energy conversion [30]. Moreover, nitrogen limitation results in the degradation of ribulose-1,5-bisphosphate (RuBP) carboxylase/oxygenase (Rubisco) for nitrogen recycling, which then leads to a diminished CO2 fixation [30]. The unbalanced carbon fixation and electron supply generated from the excessive light energy gives rise to reactive oxygen species (ROS), which accelerates photoinihibition [30,31]. The more pronounced increase of Chl a/b ratio along with the higher FvFm and ΦPSΙΙ in the 2.0 mM culture suggested a less photoinhibition and oxidative stress present therein than that in the 1.0 mM and 0.5 mM counterparts. Considering that starch accumulation in the 2.0 mM culture lagged behind the 1.0 mM culture, it is reasonable to deduce that photoinihibition should be inherently a prerequisite for starch production in T. subcordiformis.
| Time (d) | Chl a (mg/g DW) | Chl b (mg/g DW) | Chl (a+b) (mg/g DW) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.5 mM | 1.0 mM | 2.0 mM | 0.5 mM | 1.0 mM | 2.0 mM | 0.5 mM | 1.0 mM | 2.0 mM | |
| 0 | 10.3 ± 0.4 (100%) | 10.3 ± 0.4 (100%) |
10.3 ± 0.4 (100%) | 12.8 ± 0.5 (100%) | 12.8 ± 0.5 (100%) | 12.8 ± 0.5 (100%) | 23.1 ± 0.8 (100%) | 23.1 ± 0.8 (100%) | 23.1 ± 0.8 (100%) |
| 1 | 6.1 ± 0.0 (59%) | 7.1 ± 0.2 (69%) |
9.2 ± 0.8 (90%) |
7.5 ± 0.0 (59%) |
8.8 ± 0.2 (69%) |
11.2 ± 1.1 (87%) |
13.6 ± 0.0 (60%) |
15.9 ± 0.4 (69%) |
20.4 ± 1.8 (88%) |
| 2 | 4.3 ± 0.1 (42%) |
4.7 ± 0.3 (45%) |
5.9 ± 0.7 (57%) |
5.2 ± 0.1 (41%) |
5.7 ± 0.3 (45%) |
7.0 ± 0.9 (55%) |
9.5 ± 0.3 (41%) |
10.4 ± 0.6 (45%) |
12.9 ± 1.6 (56%) |
| 3 | 4.0 ± 0.0 (39%) |
4.1 ± 0.1 (39%) |
4.8 ± 0.2 (47%) |
4.8 ± 0.0 (37%) |
4.9 ± 0.1 (38%) |
5.6 ± 0.2 (44%) |
8.8 ± 0.0 (38%) |
9.0 ± 0.2 (39%) |
10.4 ± 0.4 (45%) |
Table 1. Chlorophyll changes in T. subcordiformis cultivated under limited nitrate conditions. The numbers in the parentheses represented the percentage of remained Chl levels compared to the initial day.
Non-photochemical quenching (NPQ), a parameter that reflects the heat dissipation caused by excess light energy that cannot be used for photosynthesis, is an index of efficiency of photoprotection in the photosynthetic cells [32]. It was evident that the cultures with less initial nitrate (i.e. 0.5 mM and 1.0 mM) had elevated NPQ levels on Day 2 (Figure 3d), indicating that the microalgal cells had launched a photoprotection mechanism to fight against photodamage. This was consistent with the more severe photoinihibition therein as indicated with less FvFm and Chl a/b ratio (Figure 2). It is believed that nitrogen deficiency enhances cyclic electron flow (CEF) around photosystem I (PS I), which generates a ΔpH across the thylakoid membrane and consequently activates energy-dependent NPQ (qE) [31,33]. In addition, the CEF around PS I have been shown to generate extra ATPs for the purpose of meeting the cellular energy demand (i.e. carbon fixation and starch biosynthesis), which could partially compensate for the weakened PS II activity [34,35]. Considering that the starch content and productivity peaked on Day 2 (Figures 1b and 1c) when NPQ was simultaneously enhanced (Figure 3d), it can be deduced that starch accumulation might also be facilitated by the elevated CEF around PS I which could provide ATP for carbon fixation and starch biosynthesis. Notably, as the cultivation prolonged to Day 3, NPQ showed a decline in all the cultures (Figure 3d), suggesting that the photoprotective function had been destroyed due to the severe nitrogen starvation. This was accompanied by a further decrease of photosynthetic activity and degradation of starch, as indicated by decreased FvFm (Figure 3b) and starch content (Figure 1b). The degradation of starch herein could be ascribed to the lack of energy supply from linear electron transport of PS II (decreased FvFm) and CEF around PS I (decreased NPQ). Therefore, the maintenance of photoprotective function via heat dissipation and CEF around PS I could also be important for the starch accumulation in T. subcordiformis.
T. subcordiformis was a promising microalgae for starch production under moderate nitrogen-limitation conditions. The best starch productivity of of 0.50 g L-1 d-1 and starch content of 60.5% DW were both achieved in the culture with 1.0 mM of initial nitrate. Photoinihibition was a prerequisite for starch production under nitrogen limitation in T. subcordiformis, though sufficient photosynthetic activity was also required to support the starch accumulation. The photoprotective function via heat dissipation and cyclic electron flow around PS I could also contribute to the starch accumulation. These findings provided novel clues to regulate starch accumulation in T. subcordiformis.
This work was supported by National Natural Science Foundation of China (41406177, 31500294), and the Fundamental Research Funds for the Central Universities (YJ201734).