ISSN:2321-6212
ISSN:2321-6212
Khalil Lazaar1*, Robert Pullar2, Walid Hajjaji3, Fakher Jamoussi1
1 Department of Material Sciences, Georessources Laboratory, Soliman, Tunisia
2 Department of Molecular Sciences and Nanosystems (DSMN), Ca’ Foscari University of Venice, Venezia Mestre, Italy
3 Department of Material Sciences, LABTEN Natural Water Treatment Laboratory, Soliman, Tunisia
Received: 08-Jul-2022, Manuscript No. JOMS-22-68958; Editor assigned: 11-Jul-2022, PreQC No. JOMS-22-68958 (PQ); Reviewed: 25-Jul-2022, QC No. JOMS-22-68958; Revised: 01-Aug-2022, Manuscript No. JOMS-22-68958 (R); Published: 08-Aug-2022, DOI: 10.4172/2321-6212.10.7.002.
Visit for more related articles at Research & Reviews: Journal of Material Sciences
The objective of this study is to show the adsorption power of Tunisian smectitic clay from Jebel Aidoudi from the region of El Hamma (southern Tunisia). A cationic dye (Methylene Blue: MB) and an anionic dye (Orange II: OII) are used to determine the maximum absorbability of clay. Physico-chemical characterization tests (XRD, XRF, SBET) on the clay sample were performed. The experimental results showed that the optimal contact time of adsorption is 40 min (91.35% and 51.87%, respectively, for MB and OII). An increase in the retention rate of the dyes was noted by varying the adsorbent rate from 0.06 g to 0.12 g. The experimental results showed that the retention was strongly influenced by the pH of the medium (a high adsorption rate of MB at basic pH and a high adsorption rate for OII at acidic pH). The adsorption capacities are well described by the Langmuir isotherm. The pseudo-second order model is the most reliable for determining the order of absorption kinetics of MM and OII by this smectitic clay.
Smectitic clay; Methylene blue; Orange II; Adsorption
Organic dyes are mainly used in the textile and dyeing sector. These areas are among the most water consuming industries. They generate significant pollution because of the immense amounts of effluent generated, which is heavily loaded with acid or basic dyes [1]. Eliminating these kinds of pollutants is always a challenge. Numerous studies have developed several treatment processes in order to reduce the quantities of these contaminants in aquatic environments, such as adsorption, precipitation, membrane filtration, coagulation and chemical oxidation [2]. The adsorption process is one of the methods that has shown great success for the elimination of contaminants of different kinds, in particular organic pollutants (pesticides, dyes, phenolic compounds, etc.) and heavy metals (cadmium, lead, mercury, etc.) [3]. In addition, the research and development of new naturally abundant adsorbents which are economically profitable and effective for the treatment of ecosystems is a great challenge [4,5]. Bioadsorbents such as biomass, solid agricultural waste and algae, and natural adsorbents such as soils and modified or unmodified clays have been shown to be promising materials for the trapping and removal of pollutants.
Adsorption on activated carbon is the most widely used and recommended process for the treatment of wastewater in industries. Despite its effectiveness, activated carbon remains an expensive material, and for the most part is imported, so finding new products that come from a cheap and locally available source is essential [6-8].
Clay minerals, in this case the smectite family, are among the materials most considered because of their well-known adsorption and pollutant retention properties as well as the possibility of their modification and/or functionalization [9-11].
The southern region of Tunisia is known for its vast clay deposits. In particular, clays of the smectite family, which are mainly used in the construction industry, and bentonite is applied as a waterproofing material and in the treatment of industrial effluents [12,13]. Clays are made up of minerals whose particles are essentially phyllosilicates. These form in sheets or layers, with intercalated metal ions between these layers. This type of structure leads to an interesting texture associated with very particular physicochemical characteristics, explaining the capacity of clays to allow numerous exchanges of cation and anion in the network [14,15]. In particular, smectitic clays typically consist of an anionic aluminosilicate sheet made of one tetrahedral layer topped by two or three octahedral layers, with a mobile layer of ionically attached metal cations separating these sheets.
The objective of this article is in two parts: as a first step, the physico-chemical characterization of a smectitic clay sampled from the southern region of Tunisia, and a second step, in which we studied the adsorption of cationic and anionic dyes chosen as organic pollutants (methylene blue and Orange II) by this clay. These studies were carried out as a function of several parameters in order to determine the optimal absorption conditions, and to contribute to the understanding of the behaviour of pollutant ions at the interface between clay particles and aqueous solutions.
The clay sample (AD) used in this work was collected from the Southern Atlas region. A sample of lower Coniacian-Campanian age was taken from the Meider alternation formation in Jebel Aïdoudi (El Hamma region) (Figure 1).
Methylene blue (MB) is a cationic dye, with the molecular formula C16H18N3SCl, and a maximum absorbance at 663 nm. Orange II (OII) is an anionic dye, with the molecular formula C16H11N2NaO4S, and a maximum absorbance at 486 nm.
The structures of the two dyes are shown in Figure 2 (a) and (b), respectively.
Characterisation of the clay
The determination of the mineralogical phases of our clay was carried out via X-Ray Diffraction (XRD) on a powder sample using an Philips Analytical X'PERT PRO diffractometer (with CuK α,λ=0.154056 nm) [16]. The collected data (10–80°2 range, scan rate of 0.02°/min (2)) were processed by Panalytical X’Pert Highscore software.
Chemical analysis was determined by X-ray fluorescence (XRF, Panalytical Axios Dispersive X-ray Fluorescence Spectrometer).
The specific surface area of the clay was determined from N2 adsorption desorption isotherm at -196°C using the BET (Brunauer-Emmett-Teller) method. Sieved fractions under 150 microns in size were degassed for 72 h at 70°C under high vacuum [17].
Adsorption experiments
For each experiment, the solid (clay) is brought into contact with a solution of distilled water in which the dyes have been dissolved.
For each dye (MB and OII), we treated identical volumes of the dye solution (100 ml) of initial concentration of 10 mg/l with a mass of 0.1 g of smectite clay. The mixture was stirred for one hour at room temperature (25°C.). Samples were collected at predetermined time intervals (0, 10, 20, 40, 60, 120 min) using a micropipette. The effect on the concentration of dyes was studied. The initial MB and OII dyes concentration chosen were: 10, 15, 20 and 25 ppm. The batch adsorption was performed in a thermostatic bath, by adding an amount of smectitic. We carry out the same procedure in a batch system, by adjusting the initial pH of the solutions of the dyes using the solutions of NaOH (1N) and HCl (1N), for the different pH values studied (3, 5, 7, 9 and 11). The adsorbate was then separated from the adsorbent by centrifugation for 15 min at 3000 rpm. The concentration of MB and OII was determined by measuring the absorbance at maximum wavelengths (λmax MB=663 nm, λmax OII=486 nm) by UV–Vis spectrophotometery (Shimadzu UV3100).
The percentage degree of MB and OII adsorption was calculated using the following equation 1:
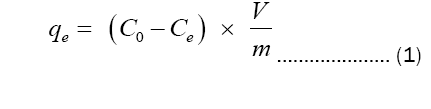
Where:
qe is the capacity at adsorption equilibrium (mg/g);
C0 is the initial concentration of the dye in the solution (mg/l);
Ce is the concentration of the dye in the solution (mg/l) at time t (t being the contact time);
V is the volume of methylene blue or orange dye II solution (ml);
m is the mass of clay (g).
Adsorption isotherms
| Adsorption model | Langmuir | Freundlich |
|---|---|---|
| Non-linear form | 1/qe=1/qm + 1/KLqmCe | log qe=log Kf + 1/n log Ce |
Table 1. Isotherm models: Langmuir and Freundlich.
Two commonly used models, Langmuir [18] and Freundlich [19], were selected (Table 1) to investigate the process of dye-clay interaction.
Where: qe is the amount of the solute at equilibrium (mg/g); Ce is the concentration of the equilibrium solute (mg/l); qm is the amount of adsorption (mg/g); KL is the Langmuir constant; Kf is the Freundlich constant which expresses the adsorption affinity (L mg-1); 1/n is the Freundlich constant which reflects the intensity of adsorption.
Adsorption kinetics
In the present work, two kinetic models were selected to study the kinetic behaviour of pollutants on the clay (AD) surface, namely the pseudo-first order kinetic model and the pseudo-second order kinetic model.
The pseudo-first order kinetic model was proposed by Lagergren [20] and assumes that the adsorption rate at time t is proportional to the difference between the amount adsorbed at equilibrium and that at time t, as shown in equation 2:
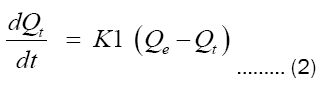
Where:
Qt: Quantity adsorbed at time t in mg/g;
Qe: Amount adsorbed at equilibrium in mg/g;
T: contact time (min);
K1: First order rate constant (min-1);
After integration, equation (2) becomes equation 3:
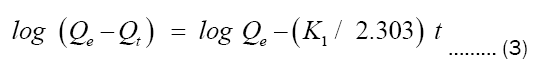
The pseudo-second order kinetic model allows characterization of adsorption kinetics by assuming rapid adsorption of the solute at high-energy sites, and relatively slow adsorption at low energy sites.
The pseudo-second order kinetic model [21] is expressed according to the following equation:
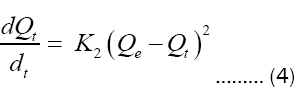
After integration, equation (4) becomes:
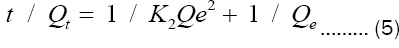
Where:
K2 = adsorption rate constants for the pseudo-second order (g mol-1 min-1).
Qe = quantity of adsorbate at equilibrium per gram of adsorbent (mg/g).
Characterization of studied clay
Mineralogical analysis shows that Jebel Aidoudi clay (AD, Table 2) contains, in addition to clay minerals, non-clay minerals mainly represented by quartz (5%), calcite (5%) and feldspar (1%). The mineralogy of the fraction less than 2 µm in diameter is essentially composed of mineral clays of the smectite (76%), kaolinite (9%) and illite (4%) type.
| Mineral phases | Weight (%) | Oxides | Weight (%) |
|---|---|---|---|
| Smectite | 76 | SiO2 | 49.53 |
| Illite | 4 | K2O | 1.49 |
| Kaolinite | 9 | Al2O3 | 18.87 |
| Calcite | 5 | Fe2O3 | 7.65 |
| Quartz | 5 | Na2O | 1.83 |
| Feldspars | 1 | P2O5 | 0.29 |
| - | - | TiO2 | 0.93 |
| - | - | MnO | 0.02 |
| - | - | CaO | 2.21 |
| - | - | MgO | 2.08 |
| - | - | LOI | 15.07 |
Table 2. Mineralogical and chemical quantification of the smectitic clay sample (AD). LOI=Loss On Ignition.
The chemical analysis of AD clay showed a dominance of SiO2 (49.53%), Al2O3 (18.87%), and Fe2O3 (7.65%), as would be expected (Table 2). The loss on ignition (LOI) is 15.07% (Table 2).
The characteristics of the porous structure of the AD clay sample are summarized in Table 3. The BET specific surface area of AD clay is around 84 m2/g due to the dominance of smectitic minerals. This value is close to that reported by Chaari et al., who studied this clay.
| Sample | SBET (m2/g) | Pore volume (cm3/g) | Pore diameter(Å) |
|---|---|---|---|
| 84 | 0.2 | 82 |
Table 3. Specific surface area, pore volume, and average pore diameter of studied clay, as determined by BET.
The nitrogen (N2) adsorption isotherms of AD are shown in Figure 3. This type of isotherm is type IV (Figure 3), with the initial part of the isotherm being attributed to monolayer-multilayer adsorption, while the section containing hysteresis loop is associated with capillary condensation taking place in the mesopores. The shape of the hysteresis loop most closely resembles the Type H3 loop, which is typically observed with aggregates of plate-like particles, such as the layered sheets of clays, which give rise to slit-shaped pores [22].Hence, this adsorbent is mesoporous (with a pore diameter exceeding 20 Å) [23]. The measured BET diameter of the pores of the clay was 82 Å.
Adsorption tests
Effect of contact time: The results of the adsorption rate of MB and OII dyes by AD clay as a function of contact time at non-adjusted pH are shown in Figure 4. The results showed that the adsorption capacity of MB and OII dyes by the clay increases rapidly initially, up to a time of 60 min, and then remains almost constant beyond this period (rapid absorption, elimination after 40 min of 90% for MB and 51% for OII). This low retention efficiency of the anionic dye OII compared to the cationic dye MB can be explained by the greater difficulty for the smectic clay (anionic sheets intercalated with cations) to adsorb anionic dyes [24].
Effect of pH: The pH plays an important role in the adsorption of the dyes studied, as shown in Figure 5. We can see from this figure that the adsorption capacity for the cationic dye MB is at a maximum at basic pH (97%). On the other hand, in the case of the anionic dye (OII), the retention was highest in an acidic medium (86%), and lower in a basic medium.
Several previous studies have been done on the adsorption of anionic dyes by clay. We can cite [1] who also showed that the optimum pH for adsorption of anionic dyes by smectitic clay was at an acidic pH.
This could be explained by the fact that in the acid state, the positive charge dominates the surface of the adsorbent. Thus, a significantly higher electrostatic attraction exists between the positive charges on the surface of the adsorbent and the negative charges on the dye [25,26].
At basic pH, despite the presence of OH- ions, we find that the capacity is higher compared to neutral pH 7, so we can say that there is always an attraction between the dyes and the adsorbent; there would be little competition between OH- ions and anions in dyes at basic pH.
For the cationic MB dye the capacity for adsorption by the clay increases with increasing pH, up to pH 9, but then shows no further increase with pH increases.
Effect of adsorbent amount: It appears through the results presented in Figure 6, that for the dye concentration of 10 mg/l, and for a contact time of 120 minutes, an increase in the clay mass from 0.06 g to 0.012 g leads to an increase in the adsorption rate of MB of the order of 84% to 93%. Likewise for the anionic dye OII, by increasing the mass used, the adsorption rate goes from 45% to 53%. This can be explained by an increase in the number of possible binding sites on the surface of the adsorbent [27].
Effect of initial dye concentration: From the results presented in Figure 7, the clay adsorption capacity of the cationic dye decreases with increasing initial concentrations (96.34% using 5 ppm and 65.26% using 25 ppm). In contrast, the clay adsorption capacity of the anionic dye increases with increasing initial dye concentrations (44.32% using 5 ppm and 76.88% using 25 ppm). This means that the saturation level is not reached and that the clay could adsorb even larger amounts. It would have been necessary to increase the dye concentrations to define the saturation threshold of the clay [10].
Adsorption isotherms: The results of the parameters characterizing the Langmuir and Freundlich models for the adsorption of MB and OII are summarized in Table 4. The values of the regression coefficients indicate that the adsorption process for both cationic (MB) and anionic (OII) dyes by AD clay can be well described by either of these two isotherm models of Langmuir and Freundlich, with linear regression coefficients R2=0.986-0.997 for Langmuir (monolayer adsorption) and 0.996 for Freundlich (multilayer adsorption), with all R2 values being very close to unity. Based on the Langmuir isotherm, the value of the MB adsorption capacity (Qm=72.45 mg/g) obtained is considerably higher than the OII adsorption capacity (Qm=7.84 mg/g). This support the observation that smectitic clays have a significantly higher retention capacity for cationic dyes than anionic dyes [1]. In this work, the values of the maximum adsorption capacities (Qm) of our smectitic clay sample obtained for MB and OII are similar to those found for many dyes in other previous works (Table 4), although lower than the values reported in ref [28] for cationic CV (Cristal Violet) and ref [29] for anionic MO (Methyl Orange).
| Types | Cationic dyes | Anionic dyes | ||||||
|---|---|---|---|---|---|---|---|---|
| MB (present study) | CV | AR | BG | OII (present study) | MO | CR | RB5 | |
| - | - | [28] | [30] | [30] | - | [29] | [10] | [9] |
| Langmuir isotherm | ||||||||
| qm (mg/g) | 72.45 | 86.54 | 60.4 | 76.9 | 7.84 | 62.34 | 11.03 | 12 |
| KL | 0.124 | - | - | - | 0.312 | - | - | - |
| R2 | 0.997 | - | - | - | 0.996 | - | - | - |
| Freundlich isotherm | ||||||||
| Kf (L/g) | 2.735 | - | - | - | 1.234 | - | - | - |
| n | 1.643 | - | - | - | 0.116 | - | - | - |
| R2 | 0.986 | - | - | - | 0.996 | - | - | - |
Table 4. Adsorption isotherm parameters for MB and OII adsorption. Comparison of the maximum monolayer adsorption of various dyes onto clay, from this work and others previously published (see references below in the table).
Where: MB: Methylene Blue; CV: Cristal Violet; AR: Allura Red; BG: Brilliant Green; OII: Orange II; MO: Methyl Orange; CR: Congo Red; RB5: Reactive Black 5.
Adsorption kinetics: The results obtained by applying the pseudo-first and second order kinetic models are collated in Table 5. The results presented in this table show that the pseudo-second order model is the most applicable in the case of the adsorption of both cationic dye (MB) and anionic dye (OII) by the smectitic clay studied (AD), which has a correlation index (R2) higher than that obtained by the pseudo-first order kinetic model in both cases. Likewise, and from the adsorption values (Qe) presented in Table 5, we note that the calculated values of Qe by the pseudo-second order model are in good agreement with those obtained experimentally. This indicates that it is chemisorption. These results are comparable with other studies which have found that the adsorption kinetics of dyes on clay supports follow the pseudo-second order [31,32].
| Dyes | Pseudo-first-order | Qe (exp) | Pseudo-second-order | ||||
|---|---|---|---|---|---|---|---|
| k1 (min-1) | Qe (mg/g) | R2 | k2 (g/mg.min) | Qe (mg/g) | R2 | ||
| MB | -0.045 | 41.81 | 0.83 | 53.17 | 0.021 | 49.53 | 0.99 |
| OII | -0.015 | 56.34 | 0.89 | 41.32 | 0.009 | 37.88 | 0.98 |
Table 5. Kinetic parameters for the adsorption of selected dyes (MB and OII) on clay.
The experimental results of adsorption of MB and OII on smectitic clay showed that the absorbability depends on the nature of the dye (cationic or anionic). This smectitic clay has better cationic dye (MB) adsorption capability than for anionic dye (OII), adsorbing 90% of MB and 50% of OII (10 ppm solutions) after 40 minutes with unmodified solution pH. The adsorption rate was influenced by the pH of the medium (high adsorption of MB in basic medium versus high adsorption for OII to acidic medium). The adsorption kinetics are in good agreement with the models of Langmuir and/or Freundlich, and a pseudo-second-order was show to be the most likely process for the two dyes studied. According to the results found from this work, we can consider that the smectitic clay of Jebel Aidoudi of the Elhamma region of southern Tunisia is a good low cost natural adsorbent material with a great capacity to adsorb MB and OII from aqueous solutions.
This work was supported by FCT-Grant SFRH/BPD/72398/2010 and by UID/GEO/04035/2013 project. This study was supported by funding from MEDYNA: “FP7-Marie Curie Action funded under Grant Agreement PIRSES-GA-2013-612572”.
The authors equally contributed for the preparation of the manuscript.
The data and material are available within the manuscript.
This is the new work and submitted to any journal first time. The manuscript is prapred as the ethical standard.
No potential conflict of interest was reported by the authors.
Yes.
Yes.