ISSN:2321-6212
ISSN:2321-6212
Muhammad Musaddique Ali Rafique123*
1 Department of Space Engineering, Massachusetts Institute of Technology, Cambridge, MA, USA
2 Department of Space Engineering, National Aeronautics and Space Administration, Merritt Island, Florida, USA
3 Department of Space Engineering, Eastern Engineering Solutions Inc, Cambridge, MA, USA
Received: 31-Jul-2023, Manuscript No. JOMS-23-108626; Editor assigned: 03-Aug-2023, PreQC No. JOMS-23-108626 (PQ); Reviewed: 17-Aug-2023, QC No. JOMS-23-108626; Revised: 24-Aug-2023, Manuscript No. JOMS-23-108626 (R); Published: 31-Aug-2023, DOI: 10.4172/2321-6212.11.3.002.
Citation: Rafique MMA. Pyrometallurgy and Electrometallurgy of Rare Earths-Part B: Cell Design, Construction, Rate Processes and Unit Operation-Neodymium Only. RRJ Mater Sci. 2023;11:002.
Copyright: © 2023 Rafique MMA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Research & Reviews: Journal of Material Sciences
Production of rare earths by metallothermic reduction and its variants is presented in an earlier study and its analysis is performed indicating major type and sequence of reactions. Diagrams required and procedures to establish them are also described. Their role and importance are also described. A comparison is also made to identify parameters of importance. This study is continuation of a previous study in which cell design to extract metals is described. Parameters to design and construct cell (metallothermic and electrothermic) are determined and used. Rate processes (heat transfer, mass transfer, charge transfer and simultaneous processes) occurring inside a cell, its operation for a specific reaction and sequence for a specific metal (Nd) are described. Outline of calculations outlining cell efficiency, output and yield are enumerated for individual (Nd, Ce, Dy) metal. Optimization and method to achieve is described. Procedures to control and production is also described. Here preliminary graphs are presented.
Rate processes; Heat transfer; Mass transfer; Efficiency; Yield
Significant research and development is being pursued to collect, refine and utilize rare earths. All but one of the 17 metallic elements (14 lanthanides and 2 associated elements) that make up rare earths is found naturally. The word "rare" in "rare earths" refers more to the difficulties of acquiring them as distinct, pure elements from natural reserves. There have been major technological advancements in separation and purification of rare earths.
Rare earths are playing increasingly significant role in low-carbon and green economy. Rare earths find their use in catalysts, lamp phosphors, rechargeable NiMH batteries, and permanent magnets. The manufacture of rare-earth materials depends on the extraction of rare-earth oxides from the ores, downstream processes, the separation into the component elements, and the processing into rare-earth metals. However, the use of rare earths in a variety of modern applications relies heavily on effective separation processes.
Several steps are involved in separation of rare earths from their mineral sources. The initial method for processing resources includes physical and chemical beneficiation techniques in addition to solvent extraction and ion exchange.
Using electrochemical reduction techniques, the rare earth oxide intermediates are converted to pure metals, based on the melting and boiling temperatures of the elements. To prepare metals with high purity, the refining process uses pyrovacuum treatment, zone melting, and electrotransport.
Significant research and development efforts are currently underway to collect, refine, and utilize rare earth elements due to their increasing significance in the low-carbon and green economy. The term "rare" in "rare earths" does not refer to their scarcity in nature but rather to the challenges associated with extracting them in their pure, distinct form from natural reserves. However, major technological advancements in separation and purification techniques have made the extraction of rare earths more feasible.
The applications of rare earths are diverse and include their use in catalysts, lamp phosphors, rechargeable NiMH batteries, and permanent magnets. The manufacturing process for rare-earth materials involves the extraction of rare-earth oxides from ores, downstream processes, separation into component elements, and processing into rare-earth metals. Effective separation processes are crucial for the widespread use of rare earths in modern applications.
Two methods that are frequently used to recover pure earth metals from primary and secondary resources are pyrometallurgy and hydrometallurgy. Due to some inherent advantages associated with hydrometallurgy, such as the potential to treat low-grade resources, easier control of wastes, and lower energy consumption, the metallurgical industry has recently been looking for hydrometallurgical processes as an alternative to pyrometallurgical treatments.
Essentially, pyrometallurgical processes involves design of furnace for melting, holding, and refining of metal oxides and compounds while electrowinning and electrorefining (after pyrometallurgy) involves design and control of electrochemical cell (room or high temperature) operated by the fundamental principles of current density, its control, distribution, and Electromotive Force (EMF). Its detail may be found in specialized texts [1-7] and literature [8-11].
This study is continuation of earlier study of author [12-14] on the subject describing extraction and refining of rare earths from their ores by metallothermic reduction.
In this, area of emphasis is cell design and rate processes (heat, mass, and activity coefficient determination) which lead towards economic production of these metals from their source. Various graphs and charts are presented lying emphasis on the efficient extraction and refining of rare earths. Nd-Fe foundry alloy is taken as example to describe construction and operation of cell. Unit operation including Piping and Instrumentation Diagrams (P and IDs) is described to explain different (Elution and electrowinning) circuits. Lastly, a future outlook is presented to calculate individual efficiency and throughput.
The separation of rare earths from their mineral sources involves several steps, including physical and chemical beneficiation techniques, solvent extraction, ion exchange, and electrochemical reduction techniques. These techniques convert rare earth oxide intermediates into pure metals based on their melting and boiling temperatures.
Pyrometallurgy and hydrometallurgy are two commonly used methods for recovering pure rare earth metals from primary and secondary resources. Hydrometallurgy offers certain advantages, such as the treatment of low-grade resources, easier waste control, and lower energy consumption, making it an attractive alternative to pyrometallurgy in the metallurgical industry.
Fundamentals and cell design
Cell design of rare earth electrowinning process depends on type of metal won (Nd [8], Sm, W, Th, U, Ce, Sc), its quantity required, production rate, type of electrolyte, concentration of metal in electrolyte and flow rate of electrolyte. Cell design primarily consists of outlining parameters of its construction based on its holding capacity.
It may be carried out in various ways such as production rate, throughput, efficiency, yield but design based on holding capacity constitutes the simplest. It is presented here. Consider a typical cell of 5 ton/day. For this, considering rectangular dimensions (as they ease out operation and maximizes yield) (Figure 1).
Cell design is a critical aspect of the rare earth electrowinning process, depending on factors such as the type of metal required (Nd, Sm, W, Th, U, Ce, Sc), the quantity needed, production rate, type of electrolyte, and flow rate of the electrolyte. Design considerations for cell construction are based on holding capacity, and examples of cell designs for a 5-ton/day capacity are provided.
Rate processes
Research progress in development and processing of rare earths may be described following [15, 16]. Density of a typical metal as a function of Concentration (c) and Temperature (T) is presented by following equation:
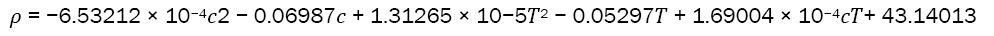
Rate processes play a vital role in the development and processing of rare earths, and their density as a function of concentration and temperature is described by a specific equation.
Solubility and dissolution of rare earth oxides in fluoride melts are also examined, and various coefficients like activity, diffusion, equilibrium separation, and effective separation coefficients are discussed to facilitate efficient electrometallurgical operations.
Solubility and dissolution of rare earth oxide in the fluoride melt: Rare earths in their oxide forms may exhibit solubility and may be dissolved in their oxide and fluoride electrolyte melts (Figure 2).
Figure 2: Solubility of rare earth oxide in fluoride molten salts [17]. Note:
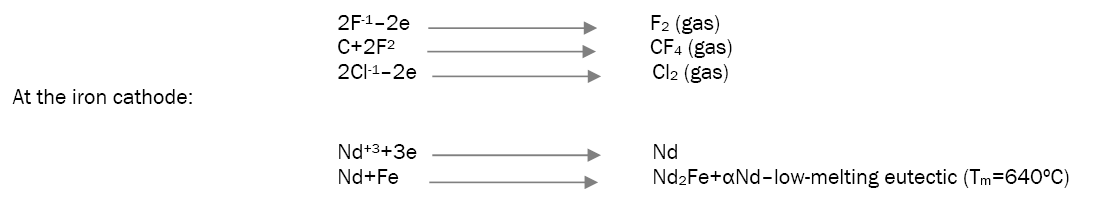
Nd2O3 solubility in fluoride melts with different NdF3-LiF compositions and at different temperatures [17]. Production of Nd-Fe foundry alloy by the electrolysis of neodymium fluoride NdF3 (Figures 3a-3c).
Figure 3: Dependence of current efficiency for Nd on the cathode current density at temperature of process t=750ºC (a) Electrolyte NdF3-LiF-BaF (b) Electrolyte BaCl2-LiCl-NdF3-LiF, (c) Electrolyte BaF2-NdF3-LiF [17].
During the electrolysis of these melts at the electrodes the following reactions are taking place: at the graphite anode [18].
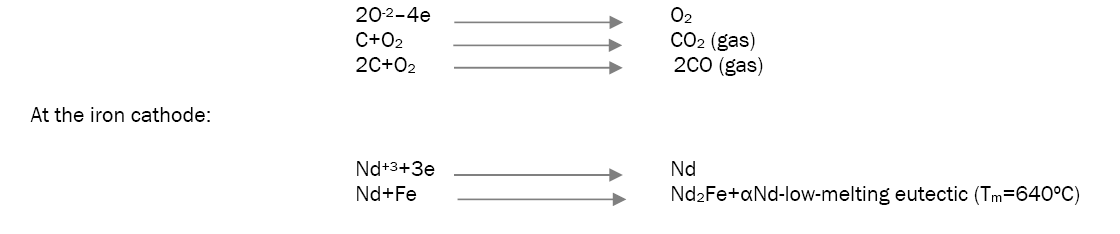
Production of Nd-Fe foundry alloy by the electrolysis of neodymium oxide: During the electrolysis of neodymium oxide in fluoride melts at the electrodes the following reactions are taking place at the graphite anode [18].
Coefficients: Following coefficients are important, must be considered and calculated for effective electrometallurgical operations. Activity coefficients, diffusion coefficients, equilibrium separation coefficients, effective separation coefficients (Figures 4a and 4b).
Figure 4: Vapor pressure as a function of temperature for various rare earths metals (a) Saturated vapor pressure of Tb, Mn and Cr, (b) Vapor pressure of other rare earth metals [19].
Vapor pressure: Vapor pressure is a crucial factor in understanding the behavior of rare earth metals at different temperatures. The saturated vapor pressure of Tb, Mn, and Cr exhibits distinct trends, providing insights into their volatility and potential applications. Additionally, the vapor pressure of other rare earth metals shows varying behaviors, which can be useful for optimizing their processing and handling in industrial applications. The comprehensive study of vapor pressure as a function of temperature contributes to the efficient extraction and refining of rare earth metals from their ores.
Cell efficiency: Cell efficiency may be calculated by measuring the concentration of reactant and product species in electrolyte. An excellent study documents these in Cerium [20]. Interested reader is referred to it for further details. Cell efficiency can be calculated by measuring the concentration of reactant and product species in the electrolyte, while mass transfer coefficients, solubility of rare earth oxides in molten fluorides, and oxide solubility determinations are important factors to consider during the process.
Mass transfer: Mass transfer coefficients, solubility of rare earth oxides in molten fluorides, oxide solubility determinations, oxide solubility diagrams. Prior to the proper oxide solubility determinations and diagram development, the equilibration time for oxide source dissolution, e.g., Dy2O3 and Dy2(CO3)3, was determined. It was determined by adding a certain amount of the oxide source (below solubility limit) and sample analysis thereafter at certain intervals. The time of equilibrium dissolution is measured by noting time at which the analyzed oxide content becomes constant. Below Figure 5 show the results obtained when Dy2O3 was added to the DyF3-LiF melts at different compositions, i.e., DyF3 (50 mol pct)-LiF (50 mol pct) and DyF3 (20 mol pct)-LiF (80 mol pct), at a working temperature of 1323 K (1050ºC). The results showed that in the case of the eutectic composition longer dissolution times than in the equimolar composition are needed, in which case, all Dy2O3 added is dissolved well within 30 minutes (Figure 6).
Figure 6: E/Volts vs ref. electrode diagrams [21].
Unit operations
Unit operations consist of drawing procedures to operate plant, optimize them, making P and IDs, setting them, calculating reactions and their sequences, and calculating efficiencies and throughput. Unit operations involve drawing procedures to operate the plant, optimizing processes, creating Piping and Instrumentation Diagrams (P and IDs), calculating reactions and their sequences, and determining efficiencies and throughput. Detailed P and IDs for a typical 5-ton/day plant are provided as examples.
Piping and Instrumentation Diagrams (P and IDs): Piping and Instrumentation Diagrams (P and IDs) for rare earths (Figures 7-9). For a typical 5 ton/day plant, example optimized P and IDs are developed. Example P and IDs for Au processing plants are shown here [22].
Figure 7: Schematic diagram showing the extraction of compounds absorbed on the elution column [22].
Figure 8: Schematic diagram of showing the process from elution column to eluant tank [22].
Figure 9: Schematic diagram of zadra elution process, where neodymium that is adsorbed on to activated carbon is desorbed from the carbon by a reversal of the adsorption kinetics [22].
Efficiency and throughput: Efficiency and throughput of individual cell comprising of circuits and P and IDs constitutes an important part of the process and an important rare determining step. These must be carefully calculated and will be presented in subsequent studies with details about each and every individual step.
Rare earths are becoming increasingly crucial in low-carbon and green technologies, and efficient electrometallurgical operations are essential for the extraction and refining of rare earth metals from their salts. Advancements in cell design, rate processes, and unit operations are driving progress in this field, and further research and development are underway to optimize these processes and promote the sustainable utilization of rare earth elements. Rare earths are playing increasingly significant role in low-carbon and green economy. Rare earths find their use in catalysts, lamp phosphors, rechargeable NiMH batteries, and permanent magnets. Various relations may be used to drive and arrive at optimum relations for efficient electrometallurgical operation of cell for efficient extraction and refining of rare earth metals from their salts. These in turn may depend on various intrinsic factors such as activity, heat, and mass transfer coefficients. These are elaborately discussed in present study. An electrowinning circuit for extraction and refining of Nd from its salts is presented. Diagrams for electrodeposition of Nd from its salts are described.