ISSN: 2320-0189
ISSN: 2320-0189
AA Warra*
Department of Biochemistry, Kebbi State University of Science & Technology, PMB 1144 Aliero, Nigeria
Received date: 12 August 2013 Accepted date: 25 October 2013
Visit for more related articles at Research & Reviews: Journal of Botanical Sciences
Hexane extracts of oil from brown and yellow Cyperus esculentus Lativum tubers was physico- chemically analyzed for quality evaluation and the following results were obtained: Acid values; 0.41±0.015 mg KOH/g and 0.48±0.014 mg KOH/g, Free fatty acids;77.00±1.0(% oleic acid) and 72.67 ±1.528(% oleic acid); Iodine values; 71.00±0.414gI2/100g and 76.67±2.517, Peroxide values; 3.80± 0.1meq H2O2 and 4.10±0.153 meq H2O2, Saponification values; 193.33±2.121 mg KOH/g and 188.33±0.707 mg KOH/g respectively. The oil yield was 26.15±3.142 % and 27.50± 5.721 respectively, while the colour was physically observed to be brown for the smaller variety (brown Cyperus esculentus tuber) and yellow for the larger variety (yellow Cyperus esculentus tuber). Justification of the cosmetic potential of the two varieties of Cyprus esculentus tubers was expatiated.
Aya, oil extracts, physicochemical, quality, cosmetics.
Cyperus esculentus Lativum is an underutilized crop of the family cyperaceae which produces rhizomes from the base and tubers that are somewhat spherical. The tubers produces high quality oil about 25.5% of its content which was implicated as lauric graded oil, non acidic stable and very low unsaturated[1]. Tigernut belongs to the sub-family Escripoidea, genus cyperus, specie esculentus, and botanical variety Lativum. Thus, its botanical name Cyperus esculentus Lativum [2]. It has many other names like; Chufa, Zulu nuts, yellow nut grass, and ground almond, edible rush and rush nuts [3]. In Nigeria, some of its native names include ‘Aya’ in Hausa, 'Imumu’ in Yoruba, ‘Ofio’ or ‘aki-hausa’ in Igbo [4]. The derivatives and benefits of Cyperus esculentus Lativum as a plant was reported[5]. This research is aimed at quality evaluation of oil from Brown and Yellow Cyperus esculentus Tubers and justify their industrial potential for cosmetic preparations.
Sample preparation
The Cyperus esculentus L .tubers which was procured from local tiger nut traders at Tawa market, Niger Republic were air-dried in the laboratory at room temperature, for a period of one week, and was then ground, using manual pestle and mortar. The dry powdered sample was then kept at room temperature in the laboratory for extraction.
Oil extraction
The extraction of oil from the tubers was carried out in a soxhlet apparatus using analytical grade hexane (n. hexane) as refluxing or extracting solvent for the work. At the completion of the extraction process the oil was recovered from the mixture by evaporating the residual extracting solvent in an oven set at 50°C and stored in the bottle. This process was repeated until a substantial quantity of oil was achieved. Each batch of extraction lasted for about 5 hours on the average [6].
Determination of Percentage Yield
The oil obtained from the extraction was transferred into a measuring cylinder which was placed over water bath for 30 min at 70°C so as to ensure complete evaporation of solvent and volume of the oil was recorded [7].
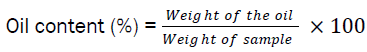
Determination of Colour
The colour of the oil samples was determined by observation using several independent competent individuals. Oil colour was correlated using colour charts [6].
Physicochemical analysis
Determination of Saponification Value [8]
2g of the oil sample was added to a flask with 30 cm³ ethalonic KOH and the flask was then attached to a condenser for 30min to ensure the sample was fully dissolved. After the sample has cooled, 1 cm³ of phenolphthalein was added and titrated with 0.2 M HCL until a pink end point has reached. The sample analysis was performed using blank. Blank was also prepared using the same reagents as the sample without the oil in it. Saponification value was calculated from the equation below:
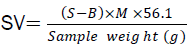
Where S= sample titre value, B= blank titre value, M= molarity of the HCL, 56.1= molecular weight of KOH.
Determination of Acid Value [9]
The acid value of the sample oil was determined by dissolving about 5.0-5.5g of the sample oil in a hot mixture of 25ml diethyl ether and 25ml 95% v/v ethyl alcohol. The hot solution was neutralized with 0.1 M NaOH using phenolphthalein as indicator.
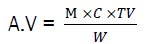
Where M = molar mass of KOH (56.1), C = concentration of KOH (0.1M), TV = titre value, W = weight of oil sample
Determination of Iodine Value [9]
About 0.26g of the sample oil was weighed into a glass stoppered flask and dissolved in 10ml cyclohexane. 20ml of Wij’s solution was added; the flask was stoppered and allowed to stand for 30 minutes in the dark at 25°C after which 20ml of 10% KI solution was added. The mixture was titrated with 0.1M Na2 S2 O3 using starch as indicator. A blank was carried out and the iodine value was calculated.
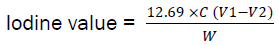
Where C = concentration of sodium thiosulphate solution, V1= volume (ml) of sodium thiosulphate solution used in blank, V2= volume (ml) of sodium thiosulphate solution used in the determination, W = weight of the sample.
Determination of Free Fatty Acid [8]
0.5g of sample oil was boiled with 5cm3 of ethanol allowed to cool and 2 drops of phenolphthalein indicator was added, then titrated with 0.1N NaOH until pink color disappear. Free fatty acid can be calculated using the expression below.
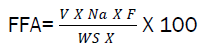
Where, V = Titre value, Na = normality of acid, F = equivalent weight of free fatty acid, Ws = weight of sample
Determination of Peroxide Value [9]
1g of the sample was weighted into a clean dry boiling tube and 1g powdered potassium iodide (KI) plus 20ml of the solvent mixture (2 volume glacial acetic acid and 1 vol. chloroform) was added. The mixture was boiled vigorously for 1minute using boiling water as the heating source. Then the boiled mixture was transferred quickly into a flask containing 20ml of 5% KI and the tube was washed twice with 25ml of distilled water into the flask. The content of the flask was titrated with 0.002M sodium thiosulphate (Na2S2O3) solution using starch as indicator. A blank was determined at the same time and condition. The peroxide value of the sample oil was calculated.
The oil yield for the brown and yellow Cyperus esculentus tubers oil were 26.15±3.142% and 27.50± 5.721% respectively. The values despite being lower than 36.75 0.50% reported for Nicotiana tabacum seed oil and recommended production of hair shampoo [10] were higher than 21.2% and 23.9% optimum yield for pericarp (peels) of avocado apples extracted by indirect and direct extraction using n–hexane respectively, which was recommended for as s a potential substitute for most oils used for cosmetics and health care production [11].
From the exploited parameters, Acid values of 0.41±0.015 mg KOH/g and 0.48±0.014 mg KOH/g were obtained for brown and yellow Cyperus esculentus tubers oil, the values were lower than the acid value of 0.81 ± 0.01 for cotton seed oil [12] 10.3 mg KOH/g for shea nut butter [13] and 2.34 ± 0.19 mg KOH/g for Luffa cylindrica (Linn.) seed oil [14] reported in literature and recommended for soap making, but relatively closer to 0.421 mgKOH/g reported for Adansonia Digitata seed oil recommended for use as an ingredient in tooth paste [15].
Free fatty acid (% oleic acid) of 77.00±1.0 and 72.67 ±1.528 (% oleic acid) were obtained the brown and yellow Cyperus esculentus tubers oil which were higher than 37.96% reported for Nigerian rubber seed oil [16] far higher than free fatty acid (oleic) obtained for bread-fruit oil (2.86%) and breadnut oil, 1.89% [17]. Oils with highly acidic free fatty acid indicates unsuitability for edible purpose except for technical purposes [16]
Iodine values obtained were 71.00±0.414 gI2/100g and 76.67±2.517 gI2/100g respectively the values are lower than Iodine value of 119.78 ± 0.81g I2/100g reported for cotton seed oil [12] but higher than 30.33 ± 2.40g I2/100g for Sponge Gourd (Luffa cylindrica) oil [14] recommended for in soap and other cosmetics. Iodine value is an indication of double bonding in the molecular structure and influences the long term stability properties of oil which is important for storage. Peroxide values of 3.80± 0.1 meq H2O2 and 4.10±0.153 meq H2O2 were obtained which are lower than 6.322mgO2/kg reported for Helianthus annuus L. seed oil [18] and 11.75 reported for fluted pumpkin [19]. The low peroxide values of the oil samples is an indication that the oils are stable and may not be susceptible to oxidative rancidity since they are produced from fresh seeds [19].
Saponification values 193.33±2.121 mg KOH/g and 188.33±0.707 mg KOH/g were obtained respectively which are lower than the saponification values of 199.42 ± 0.53 mgKOH/g for cotton seed oil [12] and 213mgKOH/g for neem seed oil [20] but higher than 116.88 ± 0.97 mg KOH/g for Cucumis melo Linn Seed oil [21] reported and recommended for cosmetic applications.
The oil extracts showed good physicochemical properties and could be useful for soap, toothpaste and other cosmetic applications.
The author wish to acknowledge the contribution of Malam Augie, the Senior Laboratory Technician, Department of Biochemistry, Kebbi State University of Science and Technology, Aliero, Nigeria.