ISSN ONLINE(2278-8875) PRINT (2320-3765)
ISSN ONLINE(2278-8875) PRINT (2320-3765)
| Bornali Bora Patowary Central Instiute of Technology, Kokrajhar, Assam, India. |
| Related article at Pubmed, Scholar Google |
Visit for more related articles at International Journal of Advanced Research in Electrical, Electronics and Instrumentation Engineering
The use of Organic Semiconductors as sensors has revolutionized modern technology in recent years due to their inherent capabilities. Organic Gas Sensors have been successfully developed in many applications to detect Humidity (both absolute and relative), various gases like Ammonia, Alcohol, Nitrogen Oxides, Chlorine, Hydrogen etc. and nitroaromatic compounds based explosive vapors. These research areas are gaining attention due to the fact that the organic semiconductors enable precise organic synthesis, low-temperature fabrication processes on variety of both rigid and flexible substrates, and straightforward miniaturization of their devices. A more appropriate selection of the optimal organic semiconductor is important by addressing the relationship of the surface chemistry of the sensor active material and its electrical response. In this article, the structural and electrical characteristics of several organic semiconductors are elaborated. Earlier works on organic gas sensors based on these active layers have been discussed. A review has been made on the past and recent works on OFETs/OTFTs for the sensing of humidity and gases like ammonia, formaldehyde, alcohol, nitrogen oxides and explosive vapors. Several breakthrough findings evolved are also being discussed.
Keywords |
| OFETs/OTFTs, Active Layer, Surface Morphology, Carrier Mobility, Threshold Voltage, Selectivity, Sensitivity, Reversibility. |
INTRODUCTION |
| Organic transistors have emerged as a revolutionary semiconductor technology because of their customizable surface chemistry, low temperature organic deposition and their much simpler fabrication processes compared to their inorganic counterparts. Conventional Silicon technology involves high-temperature and high-vacuum deposition processes and intense photolithographic patterning methods. However because of the relatively low mobility of the organic semiconductor layers, OTFTs cannot outperform single-crystalline inorganic semiconductors, such as Si and Ge, which have charge carrier mobility (μ) about three orders of magnitude higher. So, organic semiconductors are not suitable for use in applications requiring very high switching speeds. But they can be competitive as organic semiconductor devices like OFETs and OTFTs which can be fabricated easily by low-temperature deposition and solution processing methods1. In addition, the mechanical flexibility of organic materials makes them naturally compatible with plastic, paper substrates making lightweight products. Tsumura A., et al.3 reported the first organic field-effect transistor in 1986. Since then, there has been tremendous progress in both the materials’ performance and development of new fabrication techniques. OTFTs have already been demonstrated in applications such as electronic paper3-5, sensors6,7, electronic noses8, electronic skin9 and memory devices. |
ORGANIC TRANSISTORS (OFET AND OTFT) |
| Organic Field Effect Transistors consists of a doped gate, insulator, semiconductor and contacts. A doped silicon wafer provides the gate, while the insulator is grown from thick, thermally grown SiO2. A negative potential applied at the gate induces a field across the insulating oxide. Majority of hole carriers accumulate at the insulator-semiconductor interface. When a potential is applied between the source and the drain contacts, a measurable current is produced by the charge carriers collected at the drain. A gate amplified, source to drain current is produced. |
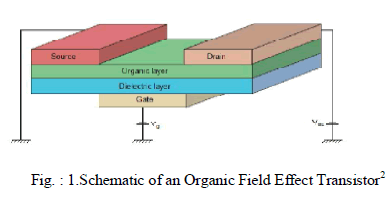 |
| As shown in Fig.1, Vg and Vds are the applied gate and source-drain voltages, respectively. The control of source-drain current in FETs via a third terminal has resulted in their widespread use as switches. |
A. ORGANIC THIN FILM TRANSISTORS (OTFTs) |
| The Thin Film Transistor (TFT) is a special kind of field-effect transistors that are made by the deposition of thin films of the different materials that make up the transistor. Like OFETs, OTFTs too inherits its design architecture from its inorganic counterpart, i.e. metal-oxide-semiconductor field-effect transistor (MOSFET). It is composed of three main components: source, drain, and gate electrodes; a dielectric layer; and the active semiconductor layer. |
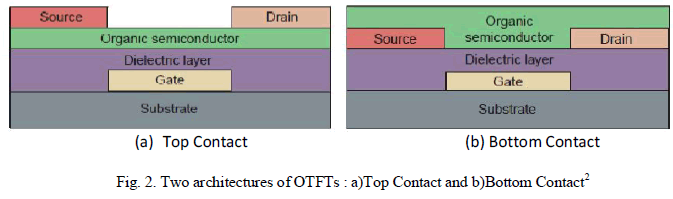 |
| Within the basic MOSFET design, there are two types of device configuration: top contact and bottom contact as shown in Fig.2. The former involves building source and drain electrodes onto a preformed semiconductor layer, whereas the latter is constructed by depositing the organic over the contacts2. |
LITERATURE SURVEY |
| Daniel E. et al.7 reported various configurations of OTFTs. They explored and reviewed the increasing applications of OTFTs right from flexible displays to sensors with a focus on recent publications. They focused on three types of sensorsbiosensor, pressur sensors and e-noses or vapour sensors. Tomohiko Mori et al.8 demonstrated remarkable influence of the surface free energy of the dielectric layer on the sensitivity of the OFET towards humidity. It indicated that the sensitivity of OFET-based gas sensors can be effected using chemically modified dielectric layers. C. Liao and F. Yan9 made a very useful and extensive review on organic semiconductor based OTFTs as chemical and biological sensors. They provided deep insight into the use of conducting polymers such as P3HT and small molecules like pentacene as the sensing layer for chemical and bio-sensors respectively. The chemical structures of various organic semiconductors and their capability as sensing material have been thoroughly discussed. M.M. Liang and Z.Bao10 provided a good review on various deposition methods for organic semiconductors, viz., Solution Processing, Organic Moelcular Beam Deposition (OMBD), Organic Molecular Beam Epitaxy (OMBE), Thermal Evaporation etc. Ongoing progress in printing and patterning of OTFTs have been surveyed. B. Crone et al.11 explored suitable properties of OTFTs for use in gas sensors. They established that chemical sensing can effectively take place through variety of mechanisms associated with semiconductor-analyte interactions and the nature of thin-film morphology. |
SELECTION OF ACTIVE LAYER FOR THE ORGANIC TRANSISTOR |
| One common feature of OFET materials is the inclusion of an aromatic or otherwise conjugated π-electron system, facilitating the delocalization of orbital wave functions. Electron withdrawing groups or donating groups can be attached that can facilitate hole or electron transport. OFETs employing many aromatic and conjugated materials as the active semiconducting layer have been reported, including small molecules such as rubrene, tetracene, pentacene, NTCDA, diindenoperylene, perylenediimides, TCNQ and conducting polymers (CPs) such as polythiophenes , polyfluorene, polydiacetylene, poly(p-phenylene vinylene). A large number of past and recent works successfully demonstrated the use of organic thin-film transistors (OTFTs) in the field of gas sensing based on conjugated polymers, oligomers, or small molecules. All these have been envisioned as a practical, low cost, efficient alternative to more traditional, mainstream thin-film transistors (TFTs) based on inorganic materials. |
OTFTs AS HUMIDITY SENSORS |
| Humidity sensors have increasingly finding their applications in industrial processing and environmental control. For manufacturing highly sophisticated integrated circuits in semiconductor industry, humidity or moisture levels are constantly monitored in wafer processing. Humidity sensors are used in domestic applications, such as air conditioner control of the living environment in buildings, cooking control for microwave ovens, and intelligent control of laundry etc. In automobile industry, humidity sensors are used in rear window defoggers and motor assembly lines. In medical field, humidity sensors are used in respiratory equipment, sterilizers, incubators, pharmaceutical processing, and biological products. In agriculture, humidity sensors are used for green-house air-conditioning, plantation protection, soil moisture monitoring, and cereal storage. In industries, humidity sensors are used for humidity control in chemical gas purification, dryers, paper and textile production and food processing19. |
| Pentacene based OTFTs have been reported as good humidity sensors. Among various organic semiconductors, pentacene has been used extensively as an active layer to make OFETs because the performance of OFETs based on pentacene is comparable and even higher than that of the hydrogenated amorphous silicon thin-film transistors. |
| The structure of pentacene as shown in Fig.3.a. is simple and its performance is highly researched. Thin films of pentacene grown through thermal evaporation in vacuum have resulted in hole mobility values up to values as high as 2.2 cm2 /Vs. The reason for the higher carrier mobility in pentacene is likely the greater molecule length, which allows more space for carrier movement. In addition to having an excellent mobility value, the on/off current ratio for pentacene OFETs can reach 108, indicating its ability to function very well as a switch. The success of linearly-fused benzene rings is likely due to the overlap of π orbitals in close-packed, highly-crystalline regions of the material, which is thought to strongly affect fieldeffect12. |
 |
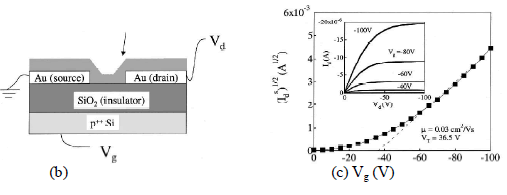 |
| Fig. 3. a) Chemical Structure of Pentacene, b)Schematic of Pentacene TFT, c) Square root of the saturation current as a function of the gate voltage in a vacuum. Inset shows the drain current vs drain voltage characteristics measured in a vacuum12. |
A. RESULTS AND DISCUSSION |
| Fig. 3 shows a pentacene based humidity sensor as demonstrated by Zhu et al.12. The saturation current of a pentacene TFT is a good parameter to monitor RH changes in the atmosphere, as it changes by as much as 80% when the RH changes from 0% to 30%. This depends on the thickness of the pentacene film and causes change to hole mobility. Humidity can affect the charge transport properties of pentacene in many different ways. It is shown that all these factors will affect the hole mobility and translate into changes in the saturation current. |
| It is shown that the fabrication process of this pentacene TFT humidity sensor is simple; it displays good reversibility, and operates at room temperature. In the study it is also investigated the behavior of pentacene OTFTs under oxygen, carbon dioxide, and nitrogen gases. The effects of these common gases on the OTFT performance are found to be very small. Therefore, the OTFT displays a reasonable selectivity towards humidity. |
| This selectivity can be further improved by coating the OTFTs with membranes that are selectively permeable to humidity. Some other gases can be detected by introducing small molecules or membranes with properly selected functional groups. For example, molecules with amine groups incorporated into an organic semiconductor film may provide selectivity to CO2 due to the acid–base interaction22,23. |
| Torsi et al.17 have dedemonstrated an NTCDA based TFT as humidity sensor the performance of which is proved to be particularly better than that of an NTCDA chemiresistor. A scheme of a typical OTFT structure is shown in Fig.4. 1,4,5,8- Napthalene-tetracarboxylic-dianhydride(NTCDA) forms highly ordered thin films when deposited by organic molecular beam deposition(OMBD) technique in ultra-high vacuum(UHV). |
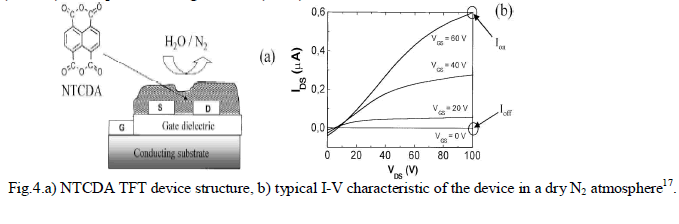 |
RESULTS AND DISCUSSION |
| As shown in fig. 4.b., due to low overlap of the π-orbitals in the direction of current flow, NTCDA TFTs exhibit mobility in the range of 10-3cm2/Vs whereas it rises upto 1-3×10-3cm2/Vs when the substrate is heated at 55oC. It is reported in the paper that Ioff is proportional to the NTCDA thin-film bulk conductivity and Ion is proportional to the field 2-D conductivity. Both Ion and Ioff behave quite differently. Ion values are at least two orders of magnitude higher than Ioff. Ion offers a larger dynamic range (Fig.4.b). Ion shows almost perfect reversibility, while Ioff exhibits hysteresis. It is experimentally demonstrated that an NTCDA based TFT can perform much better than a chemiresistor and act as multi-parameter gas sensor and distinguish different chemicals. But it is found that the performance of the NTCDA TFT is not as high as other organic transistors because of its limited dynamic range which is only of one order of magnitude. |
| In another study made by L.Torsi et al.18, several major findings emerged. When the OTFT sensor is exposed to chemical species at room temperature, four parameters, viz., the bulk conductivity of the organic thin film, the field-induced conductivity, the transistor threshold voltage and the field effect mobility are influenced. Precise measurements of all these parameters can detect the chemical analyte present. |
| Thermally evaporated polycrystalline with an average grain size of 200nm 1,4,5,8-naphthalene tetracarboxylic dianhydride(NTCDA) films of about 50 nm thickness is used here as the active layer. When the gate voltage is zero, the current that flows in the channel is proportional to the organic thin film bulk conductivity. When the gate is biased with respect to the grounded source, a surface potential at the interface between the organic layer and the gate dielectric causes the HOMO and LUMO of the organic film to bend, creating a layer less than 5nm thickness where two-dimensional transport occurs18. |
| With zero gate bias, the OTFT acts as a chemiresistor that measures the variation of the organic film conductivity upon exposure to a chemical species. |
OTFTs AS AMMONIA SENSORS |
| Ammonia gas sensors have emerged as one of the most demanding devices in the field environmental science and clinical diagnostics25,26. Several efforts have been made to develop low-cost, highly sensitive and highly selective NH3 gas sensors. Recent works have shown that device parameters like conductivity and the field-effect mobility of pentacene-based OFETs significantly get affected by ammonia (NH3) gas. |
| L. Li et al.16 devised a high-performance ammonia gas sensor based on organic field-effect transistors by growing ultrathin and microstructured organic semiconductor film.rkers demonstrate high performance OFET-based ammonia sensors with ultrathin (4-6 molecular layers) dendritic organic semiconductor microstripes prepared via dip-coating.J. Yu et al.22 reported that transistor parameters such as the saturation current (IDS), the field-effect mobility (μ), and the thresholdvoltage (VT) could be measured to understand the response of a pentacene based OFET to ammonia gas. |
| This study emphasized many factors such as the organic semiconductor molecular structures, the degree of crystallization, the grain boundaries, and the surface roughness of the organic films which can affect the sensing performance of the organic thin film20-21. The grain boundaries and the surface roughness of the organic film could play an important role in realizing the sensitivity of many gas sensors in general and OFETs based gas sensors in particular. |
| The study demonstrated that the sensor exhibits good response and recovery characteristics and sensitivity to a low NH3 concentration of 10 ppm. The air stability of the device is also investigated by storing the sensor under ambient circumstance and is characterized for 30 days to constantly detect NH3. These characterizations established a good environment stability of the OFET sensor. |
| S. Tiwari et al.25 demonstrated a poly-3-hexylthiophene (P3HT) based OFET sensor to test the sensitivity to ammonia vapor at room temperature. The use of OFETs using this kind of active materials could be limited because of their low conductivity arising out of poor mobility of charge carriers. In order to enhance the conductivity, a regioregular polymer is used here. It was shown that there is only a very small variation in the current values with prolonged exposure. The mobility and drain conductivity returned to their original values within a few(1-5)min, when device was removed from the ambiance of ammonia gas after each exposure. The value of ID at fixed gate voltage of -30V. The sensor shows significant response to ammonia at a low concentration in the range of 0.1-25 ppm at room temperature. It was revealed that when OFET sensor device was exposed in distinct concentrations of ammonia gas for few seconds under ambient condition, concentration as low as 100 ppm of ammonia gas can be detected correctly. The sensor exhibits excellent reproducibility for lower concentration of ammonia. |
OTFTs AS NOx GAS SENSORS |
| Highly pollutant and toxic gas like NO2 in the environment has emerged as a major issue to public health31 with the increase in automobile fuel vehicles and chemical industries. More disturbing fact is that NOx emanated from diesel once out in the air forms more harmful ozone gas.Several NO2 solid gas sensors reported in the past. But due to high working temperatures made these sensors less popular. Organic TFTs for NO2 gas sensing, hence, gained much attention. Several such sensors have been successfully demonstrated with good results. |
| Phthalocyanines (Pcs) and structurally related compounds have been evolved as effective NO2 gas sensors. Phthalocyanine (Pc) is a conjugated heterocyclic 18-π electron containing compound. One of the important advantages of Pcs over other organic materials is their thermal and chemical stability. Metallophthallocyanines (MPcs) exhibit high sensitivity towards lower concentration of oxidizing and reducing gases such as NO2, ozone, chlorine and bromine25. |
| Opera et al.28 demonstarted a suspended gate field effect transistor and established that CuPc is appropriate choice for NO2 detection at room temperature with low power consumption. T.A. Temofonte et al.29 devised a NiPc based thin film transistor for NO2 sensing. A highly sensitive and selective NO2 gas sensor is devised by B. Bott et al.30. It is described that the sensor is suitable for detecting and measuring NO2 at concentrations from 1 ppb to 10 ppm in air. The device is based on the electrical conductivity changes effected by the adsorption of NO2 on films of lead phthalocyanine at temperatures above 100 °C. |
| 9,10-ter-anthryleneethynylene (D3A) is an amorphous organic semiconductor having good stability in air. This has been investigated as NOx OTFT sensor by F. Marinelli32. The D3A OTFT operated at room temperature exhibited different sensitivity to NO and NO2. Interfering gases like carbon monoxide and hydrogen sulphide are introduced in the environment and the NO2 detection was found down to 250 ppb. |
| D3A OTFT exposed to different concentrations of NO gas within 0.6–5ppm gave sensing responses similar to those observed for NO2. The average current changes per unit concentration were significantly smaller for NO compared to NO2 because of strong electron acceptor character of NO2 while NO is a weaker electron withdrawing molecule. The OFET shows a fast response characteristic at room temperature and no heating is required to attain a full baseline recovery. Interactions at defect sites causes increase in IDS. It is established in the study that D3A OFET provided two orders of magnitude higher field-effect mobility than the similar active layers of poly(triarylamine). The experimental data shows a low sensitivity towards CO and H2S. Response repeatability(relative standard deviation) was in the range 2-12% range similar to thiophene based OTFTs exposed to alcohols33. |
| The influence of channel parameters like its length to width ratio and the thickness of the dielectric layer on the gas-sensing characteristics of NO2 gas sensors for OTFTs were investigated in a paper by Y.Jiang et al.34. The results showed that by setting optimized device parameters the sensitivity and response time of sensors can be improved. The bottom-contact TFT sensor with the α-Sexithiophene (α-6T) film incorporated into a sensitive layer was observed to respond strongly to the trace nitrogen dioxide (NO2) vapor ranging from 0.2 to 1ppm at room temperature. The vapor sensing capabilities of OFETs to some analytes can be further improved by adding a ‘sensitiser layer’ as studied in the thesis paper by Lee Hague, 201235. |
| The α-6T was deposited by vacuum deposition as the sensitive layer for the detection of NO2, and the CuPc thin film was chosen as the active layer for the test of hydrogen sulfide (H2S). The thickness of the sensitive thin film was about 90 nm. |
| The results showed that OTFTs with channel Width to Length ratio between 160 and 640 and the 195 nm dielectric layer provided the optimized gas-sensing properties. The OTFTs based on α-6T sensitive film can be used to detect traces of NO2 with the least detection limit as 0.2ppm. |
OTFTS AS FORMALDEHYDE GAS SENSORS |
| Formaldehyde(HCHO) is a commonly used chemical compound that exists in various forms and at room temperature. It is a colorless, distinctive, strong, pungent smelling and flammable gas, easily soluble in water, alcohol, and ether and used in number of industries for various purposes. As per “11th report on carcinogens”, formaldehyde is classified as “reasonably anticipated to be a human carcinogen”. |
| Dan et al.36 devised an OTFT sensor based on composite film of P3HT(poly-3-hexylthiophene regioregular) and ZnO nanoparticles as the active layer to detect formaldehyde. Different ratios of P3HT and ZnO are taken at 3:1, 1:1 and 1:3 ratios by spray-deposition technology. It is demonstrated that the 1:1 ratio showed the best response and recovery characteristics. The OTFT sensor with the P3HT/ZnO ratio of 1:1 composite film revealed largest change in threshold voltage and carrier mobility. The surface morphology has significant influence on the sensing properties of the OFET. The scanning electron microscopy(SEM) images with 1:1 ratio showed more efficient contact sites with HCHO. The tested HCHO concentration in the experiment was 100 ppm with N2 as the carrier gas. The electrical properties, sensing properties of the sensor depict dependence on the P3HT/ZnO composite and 1:1 ratio was established as the optimized one. |
OTFTS AS ALCOHOL GAS SENSORS |
| M.C. Tanese et al.37 designed a pentacene based bottom-contact OTFT to detect alcohol. It is demonstrated that the sensitivity is improved when the device works in the accumulation mode. |
| In another paper38, a bottom contact pentacene based organic thin film transistor (OTFT) was fabricated to sense for alcohol vapor. Pentacene thin film acting both as gas sensing layer and the active layer was formed by vacuum evaporation and was characterized by atomic force microscopy (AFM) and X-ray diffraction (XRD). The results show that the film is highly ordered and has a polycrystalline morphology. The current-voltage characteristics of the OTFT were obtained and examined. The drain-source current in the saturation region found to change when the OTFT was exposed to alcohol vapor compared to that under an exposure to nitrogen gas. The drain-source current(IDS) emerged as a key parameter to monitor chemical species. The paper established that such OTFTs can act as promising device as a novel class of chemical sensors. |
OTFTS AS EXPLOSIVE GAS SENSORS |
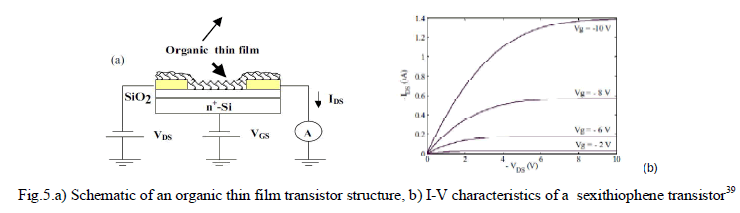
| In a paper by E. Bentes et al.39, a prototype sensor is described to respond to nitroaromatic compounds emerging in an environment containing a landmine. Here, field effect transistors (FETs) based on α-sexithiophene were investigated as sensors for detecting 2,4,6-trinitrotoluene (TNT) vapors. When vapors of nitroaromatic compounds bind to thin films of organic materials and thus forming the transistor channel, the conductivity of the thin film increases and changes the transistor electrical characteristic. |
 |
 |
RESULTS AND DISCUSSION |
| A typical sexithiophene(6-αT) based organic TFT device is shown in Fig. 5.a. All the electrical measurements were carried out in vacuum (10-6mBar).The transistors used in this study exhibit good characteristics with a low off current and field effect mobility in the order of 10-2 cm2/Vs. The output characteristics (IDS-VDS) show current saturation as well as negligible contact resistance as shown in fig.5.b. |
| When the transistor is exposed to 4-nitrotoluene vapors, the (IDS) current increases quite rapidly and reaches a final value that is more than double the initial current magnitude. It was also observed the changes are irreversible even on vacuum pumping. Also the devices remain insensitive to any further exposures to TNT vapors. This establishes the fact that TNT molecules interact strongly and in a permanent way with the organic layer.In the study, it is observed that transistor electrical properties degrade when exposed for longer periods of time (months) to air, though sexithiophene transistors can still respond to TNT vapours. |
| Changes in the sensor design are expected to improve the minimum detection limit of the sensor and improve the low term stability in atmospheric environment. The concentration of TNT present in the air over a landmine is likely to be in the femtograms per ml of air. It is not possible yet to reach this sensitivity. In future, similar devices can be produced at low cost in flexible substrates with large active areas, so that they may reach the sensitivity to detect gases in buried landmines too. There is enough research potential to adapt such sensors to sense other substances including pesticides. |
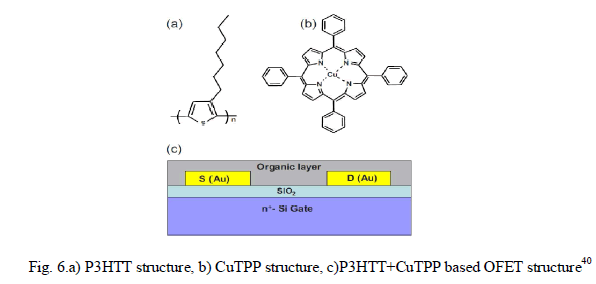 |
| In another paper40, the organic layer of the OFET sensor was structured as a composite film of poly(3-hexylthiophene) P3HT and CUII tetraphenylporphyrin(CuTPP)(shown in Fig.6.) to detect nitro-based explosive compounds. Two parameter were considered for sensing – i) drain current(ION) and ii)linear-region conductance. |
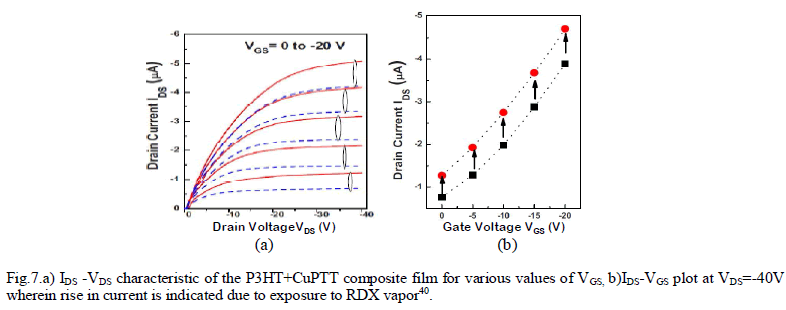 |
RESULTS AND DISCUSSION |
| Fig. 7.b. shows the output characteristics which were obtained both for with and without exposure to RDX vapors. Drain current characteristics(Fig.7.a) were obtained at various gate voltages which depict significant rise in drain current after the sensor being exposed to RDX vapor. The conductance of the sensor film(Fig.7.b) also is changed by 10%-15% after the exposure. The paper exploited some previus findings regarding the formation of molecular complexes between metalloporphyrins and various acceptors, superior sensor response is achieved due to coordinate bonding between metalloporphyrin molecules and the nitro group compounds as well as π-stacking of porphyrins and aromatic rings of P3HT polymer. |
CONCLUSION |
| In this paper, several past and recent works on organic based gas sensors are discussed taking into account several important factors that can influence the device performances. Gas sensors based on small molecules and conducting polymers(CP) are reviewed. The selection of semiconductor material as the sensing layer, their surface morphology, composition and deposition techniques are highlighted. The dependence of device output on electrical and sensing properties of the sensors are studied. The aspects relating to better performance of OFET based sensors over their resistive counterparts are also being focused. The major findings of the above studies have proved the fact that organic semiconductors possess enormous potential to detect a large no. of gas analytes at a very low ppm and at ambient condition involving attractively low cost. The possibility of improving the sensitivity, selectivity, stability and reversibility of the sensing devices over standard resistor-type configuration and their future scope have been discussed. There is enormous research scope to extend the dimesion of applicability of organic semiconductors in the field of gas sensing by tuning the organic layer as desired. Future lies in sensing multiple analytes together using sensor arrays like e-noses and e-skin. |
References |
|