ISSN: 2320-0189
ISSN: 2320-0189
Alemayehu Hailu*, Tajudin Aliyi, and Bayoush Birke
Ethiopian Institute of Agricultural Research, Ambo Plant Protection Research Center, Ambo, Ethiopia
Received Date: 01/03/2018 Accepted Date: 05/03/2018 Published Date: 12/03/2018
Visit for more related articles at Research & Reviews: Journal of Botanical Sciences
Common leaf rust (Puccinia sorghi Schw) and Turcicum leaf blight (Exserohilum trurcicum) is the major foliar fungal diseases of maize in Ethiopia causing yield losses in the range of 12% to 61% rely up on the genotypes. Screening was done on 178 (106 quality protein maize and 72 normal maize lines) maize inbred lines against Common leaf rust (CLR) and Turcicum leaf blight (TLB) diseases in order to know the reaction of those maize lines for two consecutive years. The experiment was conducted at Ambo plant protection research center (TLB and CLR) and Bako agricultural research center (TLB only), on station experimental fields. Out of 178 maize inbred line, 105 (53 quality protein maize and 52 normal maize lines) were evaluated for CLR; and 73 (53 quality protein maize and 20 normal maize lines) were evaluated for TLB. A randomized complete block design was used. Artificial inoculation was made twice a week for three continuous weeks, when plants were 30-45 cm high (4-5 leaf stages). Among 73(53 quality protein maize and 20 normal maize lines) maize lines, resistant and susceptible responses were recorded on 42 (33 quality protein maize and 9 normal maize lines) and 3 (1 quality protein maize and 2 normal maize lines) lines for TLB disease, respectively. Out of 105 (53 quality protein maize and 52 normal maize lines) maize lines, resistant and susceptible responses were recorded on 33 (11 quality protein maize and 22 normal maize lines) and 4 (quality protein maize only) lines for CLR disease, in that order. Those selected resistance maize lines from this screening will be used in breeding program and finding of resistant maize lines for both diseases should be continued using modern screening tools as well as techniques in addition to this conventional method.
Common leaf rust, Puccinia sorghi Schw, Turcicum leaf blight, Exserohilum trurcicum, Resistant response, Susceptible response, Maize lines
Maize (Zea mays L.) is an important staple food crop and provides raw materials for the livestock and many agro-allied industries in the world [1]. It is a staple food for several million people in the developing world where they derive their protein and calorie requirements from it.
Maize is among the leading cereal crops selected to achieve food self-sufficiency in Ethiopia [2]. Although, improved cultivars have been largely included in the national extension package, the national average yield of maize is only 3.45 tons/ha, which is far below the world average of 5.5 tons/ha [3].
Amongst the major constraints limiting maize productivity are abiotic (moisture, soil fertility, frost, etc.,) biotic stresses such as diseases, insect pests, and weeds [4]. These constrains vary among growing areas and between cropping seasons. For example, disease epidemics and insect pest out break frequently occurs a result of the warm climate and/or high rain fall common to maize production zones [5]. Among biotic factors, foliar diseases such as turcicum leaf blight (Exserohilum trurcicum) and common rust (Puccinia sorghi Schw) are generally among the important constraints in tropical maize production [6,7]. In Ethiopia, the two diseases can cause yield loss in the range of 12.0% to 61.0 percent depending up on the genotype. Previously these diseases were limited to specific areas and varieties, but currently the disease become very important almost in all maize growing agro-ecologies due to climate change and pathogens virulent and/or avirulent shifts.
Although there are some commercial maize cultivars available with some level of resistance to these diseases, more farmers need to adopt resistant maize varieties in order to with stand future CLR and TLB out breaks in Ethiopia. These diseases are often difficult to control since their occurrence year after is less predictable because of their high dependence on weather. The majority of small scale farmers, in most cases, do not control these diseases due to limited access to fungicides and unaffordable prices. Foliar diseases also occur mainly after the tasseling stage of maize, making them difficult to control with fungicides in the field. Improvement of genetic resistance to foliar diseases through understanding of disease reactions is essential for parental selection as well as resistance hybrid development [8]. The development of maize cultivars with enhanced levels of disease resistance will be sustainable and effective for increased maize yields, especially in the smallholders farming Sectors. This can be achieved through continuous screening of maize lines against Common leaf rust and Turcicum leaf blight. CLR and TLB can be effectively controlled by growing resistant varieties. Genetic resistance is the safest and best control strategy for resource-poor farmers in addition to being profitable option for farmers that can multiply seed [9]. Thus, the objective of this study was to evaluate the reaction of maize lines against CLR and TLB under field conditions with artificial inoculation.
Study Area Description
Experiment was done at Ambo and Bako Agriculture Research Center for two consecutive years (2014 – 2015 main cropping seasons). Ambo Plant Protection Research Center (APPRC) is located at 080 96' 885'' N latitude and 370 85' 923'' E longitude and at an altitude of 2147 m.as. l. The annual average temperature and rain fall is 27.54°C and 1077.68 mm, respectively. Bako is located at an altitude of 1650 m.a.s.l, 9°06΄ north latitude and 37°09΄ east longitude. Average annual rainfall at this location is 1246 mm.
Maize Field Evaluation and Plating Materials
One hundred and seventy eight maize planting materials were evaluated against Common Leaf Rust (CLR) and Turcicum Leaf Blight (TLB). These maize planting materials were obtained from the national high land maize breeding programs of Ambo plant protection Research center. Among 178 maize lines, 106 lines were quality protein maize (QPM) and the remaining 72 maizes were normal maize (Non- QPM) lines.
The experiment was conducted for two consecutive years (2015 and 2016 growing seasons) at Ambo (TLB and CLR) and Bako (TLB only) screening nursery sites. Maize lines were evaluated against CLR and TLB diseases. The treatment was arranged following a randomized complete block design (RCBD) with three replications for two disease types, separately. The plots were ploughed with tractor and disc harrowed twice before planting. The distance between rows and plants were 75 cm and 25 cm, respectively. All plots were planted by hand with two seeds per hole. Inorganic fertilizer (Dap &Urea) and all agronomic practices were applied based on the area recommendations.
Inoculation and Disease Assessment
Inoculation ways
The TLB pathogens were isolated by collecting diseased maize leaf lesions from experimental sites and placing in a moist chamber. After two-three days newly formed spores on the surface of the lesions was picked up with the help of fine flattened needle under a dissecting microscope placed in a droplet of sterile water and streak across the surface hardened, acidified water agar in petri-plates. After 6 hrs the spores start to germinate, and it was cut out of the agar and transferred to hard, acidified PDA. After two weeks of incubation at 20°C-25°C, this culture was transferred to fresh plates of acidified PDA for multiplication. When the fungus growth was covered the surface of petri-plate fully, the cultures were ready for use. The spore (TLB) suspension at 60,000 spores/ml was applied in the whorl using atomizer hand sprayers. Inoculation was made twice a week for three weeks, when plants were 30-45 cm high (at 4-5 leaf stages). After inoculation, water was sprayed with hand atomizer to create favorable conditions for pathogen germination.
Inoculum (rust) was collected naturally infected leaves showing large number of pustules. Collection of rust uredospore was done by lightly tapping the leaves in to a cup or a suitable container. The spores were dried and kept in tightly sealed glass jars and stored at minus 20°C. Maize plants under field conditions were inoculated first time at around 6-8 leaf stage and it repeated within 2 weeks at seven days interval. Rust spore suspension at 60,000 spores/ml was prepared and applied in the whorl using hand atomizer. To avoid spores clump together on the upper surface of the water, the spore suspension was agitated (stirred) continuously and tween 20 was added in the solutions.
Disease assessment
Disease assessment commenced 7 days after inoculation. Six assessments were made at 7 days intervals from four central tag maize plants with visual observations and the following parameters were recorded:
Disease severity estimation
Maize lines were phenotyped for TLB and rust severity when the diseases are appeared using standard 1-5 scale, 1 being complete resistant and 5 being the complete susceptible. Based on this rating scale over two years, maize lines were categorized into four groups namely, resistant (R) genotypes with a score <2.0; moderately resistant (MR) 2.1-3.0; moderately susceptible (MS) 3.1-3.5 and highly susceptible (S) >3.5. Severity scores were converted to percept disease index (PDI) as described by Wheeler (1969) using the formula below [10].
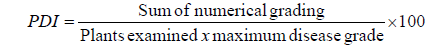
Area under disease progress curve (AUDPC)
AUDPC (% day) was calculated from severity after conversion of percept disease index (PDI) and it was recorded 6 times at seven-days interval starting from one set of disease after artificial inoculation for each year. Disease severity was recorded from 10 randomly selected and tagged plants in each plot for AUDPC calculation. AUDPC was calculated using the formula suggested by Wilcoxson et al. [11].
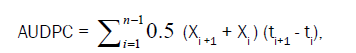
Where, Xi is the cumulative disease severity expressed as a proportion at the ith observation; ti is the time (days after planting) at the ith observation and n is total number of observations.
Data Analysis
Analysis of variance (ANOVA) was used for disease data as randomized block design (RCBD) and following the procedure described by Gomez and Gomez using SAS computer software. Mean separation was done based on LSD at 5% probability level. Disease data was analysed after checking for good fitness to ANOVA.
Among 33 tested quality protein maize (QPM) lines, totally 26 (78.8%) lines were showed resistant responses for Turcicum leaf blight at Ambo and Bako in 2015 (Table 1). Among 26 recorded quality protein resistant maize lines, 14 (53.8%) resistant maize lines were obtained from both Bako and, whereas 12 (46.2) resistance quality protein maize lines were got only from Ambo screening site., in 2015 (Table 1). Moderately responses were recorded at Ambo and Bako on seven and eighteen maize lines in 2015, respectively. Only one QPM maize line showed susceptible response at Bako experimental site (Table 1).
| S.no | Maize lines | Severity (1-5 scales) | Maize line responses | ||
|---|---|---|---|---|---|
| Ambo | Bako | Ambo | Bako | ||
| 1 | [KIT/SNSYN[N3/TUX]]c1F1-##(GLS=1.5)-31-17-1-1 /CML144(BC2)-31-14-1-3-2-2-#-1-1 | 2.3 | 2.04 | MR | MR |
| 2 | [KIT/SNSYN[N3/TUX]]c1F1-##(GLS=2)-29-35-2-3/CML144(BC2)-29-24-1-2-1-3-#-3-1 | 2.3 | 2.5 | MR | MR |
| 3 | [KIT/SNSYN[N3/TUX]]c1F1-##(GLS=2)-29-35-2-3/CML144(BC2)-29-24-1-3-1-1-#-2-1 | 1.5 | 1.9 | R | R |
| 4 | [KIT/SNSYN[N3/TUX]]c1F1-##(GLS=2)-29-35-2-3/CML144(BC2)-29-24-1-3-2-2-#-1-1 | 2.2 | 2.04 | MR | MR |
| 5 | [KIT/SNSYN[N3/TUX]]c1F1-##(GLS=2)-29-35-2-3/CML144(BC2)-29-24-1-3-3-1-#-1-1 | 1.9 | 2.8 | R | MR |
| 6 | [KIT/SNSYN[N3/TUX]]c1F1-##(GLS=2)-29-35-2-3/CML144(BC2)-29-24-1-3-3-3-#-2-1 | 1.7 | 1.9 | R | R |
| 7 | [POOL9Ac7-SR(BC2)]FS211-1SR-1-1-1-#/CML144(BC2)-14-21-1-3-2-2-#-1-1 | 1.8 | 1.9 | R | R |
| 8 | [POOL9Ac7-SR(BC2)]FS211-1SR-1-1-1-#/CML144(BC2)-14-21-2-1-4-1-#-2-1 | 1.7 | 1.8 | R | R |
| 9 | [POOL9Ac7-SR(BC2)]FS211-1SR-1-1-1-#/CML144(BC2)-14-21-2-4-5-2-#-4-1 | 1.8 | 2.5 | R | MR |
| 10 | [POOL9Ac7-SR(BC2)]FS211-1SR-1-1-1-#/CML144(BC2)-14-8-4-2-1-3-#-1-1 | 1.9 | 2.5 | R | MR |
| 11 | [POOL9Ac7-SR(BC2)]FS211-1SR-1-1-1-#/CML144(BC2)-14-8-4-2-2-1-#-2-1 | 1.9 | 2.4 | R | MR |
| 12 | [POOL9Ac7-SR(BC2)]FS211-1SR-1-1-1-#/CML144(BC2)-14-8-4-2-2-4-#-3-1 | 2.4 | 2.2 | MR | MR |
| 13 | [POOL9Ac7-SR(BC2)]FS211-1SR-1-1-1-#/CML144(BC2)-14-8-4-2-3-3-#-1-1 | 1.9 | 2.5 | R | MR |
| 14 | [POOL9Ac7-SR(BC2)]FS211-1SR-1-1-1-#/CML144(BC2)-14-8-4-2-4-1-#-1-1 | 1.5 | 2.2 | R | MR |
| 15 | [POOL9Ac7-SR(BC2)]FS211-1SR-1-1-1-#/CML144(BC2)-14-8-4-3-2-4-#-1-1 | 1.5 | 1.7 | R | R |
| 16 | [POOL9Ac7-SR(BC2)]FS211-1SR-1-1-1-#/CML144(BC2)-14-8-4-3-3-4-#-2-1 | 1.4 | 1.5 | R | R |
| 17 | [POOL9Ac7-SR(BC2)]FS211-1SR-1-1-1-#/CML144(BC2)-14-8-4-3-4-2-#-3-1 | 1.5 | 1.7 | R | R |
| 18 | [POOL9Ac7-SR(BC2)] FS48-1-1-1-1-1-#/CML144(BC2)-6-25-2-2-4-1-#-1-1 | 1.5 | 1.7 | R | R |
| 19 | [POOL9Ac7-SR(BC2)] FS48-1-1-1-1-1-#/CML144(BC2)-6-25-2-2-4-1-#-5-1 | 1.6 | 1.9 | R | R |
| 20 | [POOL9Ac7-SR(BC2)] FS48-1-1-3-1-#/CML144(BC2)-15-8-1-1-3-2-#-1-1 | 1.4 | 1.7 | R | R |
| 21 | [POOL9Ac7-SR(BC2)] FS59-2-2-1-1-#/CML144(BC2)-9-5-2-1-3-1-#-4 | 1.7 | 1.9 | R | R |
| 22 | [KIT/SNSYN[N3/TUX]]c1F1-##(GLS=2.5)-32-1-1-#/CML176BC1F1-12-1-3-2-1-#-1-B | 1.6 | 1.7 | R | R |
| 23 | [KIT/SNSYN[N3/TUX]]c1F1-##(GLS=2.5)-32-1-1-#/CML176BC1F1-12-1-3-4-2-#-1-B | 1.5 | 2.1 | R | MR |
| 24 | [POOL9Ac7-SR(BC2)] FS60-2-1-1-1-#/CML176(BC2)-1-3-2-3-3-#-2-B | 2.2 | 3.6 | MR | S |
| 25 | [POOL9Ac7-SR(BC2)] FS60-2-1-1-1-#/CML176(BC2)-1-3-2-3-4-#-1-B | 1.8 | 2.6 | R | MR |
| 26 | [POOL9Ac7-SR(BC2)] FS60-2-1-1-1-#/CML176(BC2)-1-3-2-3-5-#-1-B | 1.7 | 2.5 | R | MR |
| 27 | [POOL9Ac7-SR(BC2)] FS60-2-3-1-3-1-#/CML176(BC2)-3-1-3-3-3-#-1-B | 1.8 | 1.5 | R | R |
| 28 | SADVLA/CML154 BC2F54-4-1-B-#-4-# | 2.8 | 2.8 | MR | MR |
| 29 | SADVLA/CML154 BC2F41-1-12-B-#-#-# | 1.4 | 2.4 | R | MR |
| 30 | SADVLA/CML154 BC2F37-2-1-B-#-#-# | 1.6 | 1.7 | R | R |
| 31 | P502 SR/CML384X176ÃÆâÃâââ¬Ãâæ.98-2-1-2 BC2F4-1-3-B-#-#-# | 1.6 | 2.3 | R | MR |
| 32 | CML176 | 2.5 | 2.4 | MR | MR |
| 33 | CML491 | 1.6 | 2.5 | R | MR |
Table 1. Reaction of maize (QPM) lines for TLB disease at Ambo and Bako, in 2015.
Among 33 QPM lines, 6 (18.2%) lines showed resistant responses for Common leaf rust at Ambo, in 2015 (Table 2). From 33 QPM maize lines, 18 (54.5%) lines showed moderately resistant (MR) responses at ambo in the same experimental season. Moderately susceptible (MS) responses were recorded on 6 maize lines. Whereas, three QPM maize lines (SADVLA/CML154 BC2F54- 4-1-B-#-4-#, P502 SR/CML384X176…..98-2-1-2 BC2F4-1-3-B-#-#-# and CML176) were showed susceptible (S) responses for common leaf rust at Ambo, in 2015 (Table 2).
| S.no | Maize lines | Severity (1-5 scales) | Line response |
|---|---|---|---|
| 1 | [KIT/SNSYN[N3/TUX]]c1F1-##(GLS=1.5)-31-17-1-1 /CML144(BC2)-31-14-1-3-2-2-#-1-1 | 3.2 | MS |
| 2 | [KIT/SNSYN[N3/TUX]]c1F1-##(GLS=2)-29-35-2-3/CML144(BC2)-29-24-1-2-1-3-#-3-1 | 3.2 | MS |
| 3 | [KIT/SNSYN[N3/TUX]]c1F1-##(GLS=2)-29-35-2-3/CML144(BC2)-29-24-1-3-1-1-#-2-1 | 3.2 | MS |
| 4 | [KIT/SNSYN[N3/TUX]]c1F1-##(GLS=2)-29-35-2-3/CML144(BC2)-29-24-1-3-2-2-#-1-1 | 2.1 | MR |
| 5 | [KIT/SNSYN[N3/TUX]]c1F1-##(GLS=2)-29-35-2-3/CML144(BC2)-29-24-1-3-3-1-#-1-1 | 2.4 | MR |
| 6 | [KIT/SNSYN[N3/TUX]]c1F1-##(GLS=2)-29-35-2-3/CML144(BC2)-29-24-1-3-3-3-#-2-1 | 2.2 | MR |
| 7 | [POOL9Ac7-SR(BC2)]FS211-1SR-1-1-1-#/CML144(BC2)-14-21-1-3-2-2-#-1-1 | 2.2 | MR |
| 8 | [POOL9Ac7-SR(BC2)]FS211-1SR-1-1-1-#/CML144(BC2)-14-21-2-1-4-1-#-2-1 | 2.3 | MR |
| 9 | [POOL9Ac7-SR(BC2)]FS211-1SR-1-1-1-#/CML144(BC2)-14-21-2-4-5-2-#-4-1 | 2.2 | MR |
| 10 | [POOL9Ac7-SR(BC2)]FS211-1SR-1-1-1-#/CML144(BC2)-14-8-4-2-1-3-#-1-1 | 2.5 | MR |
| 11 | [POOL9Ac7-SR(BC2)]FS211-1SR-1-1-1-#/CML144(BC2)-14-8-4-2-2-1-#-2-1 | 3.2 | MS |
| 12 | [POOL9Ac7-SR(BC2)]FS211-1SR-1-1-1-#/CML144(BC2)-14-8-4-2-2-4-#-3-1 | 1.6 | R |
| 13 | [POOL9Ac7-SR(BC2)]FS211-1SR-1-1-1-#/CML144(BC2)-14-8-4-2-3-3-#-1-1 | 2.2 | MR |
| 14 | [POOL9Ac7-SR(BC2)]FS211-1SR-1-1-1-#/CML144(BC2)-14-8-4-2-4-1-#-1-1 | 2.2 | MR |
| 15 | [POOL9Ac7-SR(BC2)]FS211-1SR-1-1-1-#/CML144(BC2)-14-8-4-3-2-4-#-1-1 | 2.1 | MR |
| 16 | [POOL9Ac7-SR(BC2)]FS211-1SR-1-1-1-#/CML144(BC2)-14-8-4-3-3-4-#-2-1 | 2.1 | MR |
| 17 | [POOL9Ac7-SR(BC2)]FS211-1SR-1-1-1-#/CML144(BC2)-14-8-4-3-4-2-#-3-1 | 1.5 | R |
| 18 | [POOL9Ac7-SR(BC2)]FS48-1-1-1-1-1-#/CML144(BC2)-6-25-2-2-4-1-#-1-1 | 1.8 | R |
| 19 | [POOL9Ac7-SR(BC2)]FS48-1-1-1-1-1-#/CML144(BC2)-6-25-2-2-4-1-#-5-1 | 1.9 | R |
| 20 | [POOL9Ac7-SR(BC2)]FS48-1-1-3-1-#/CML144(BC2)-15-8-1-1-3-2-#-1-1 | 2.2 | MR |
| 21 | [POOL9Ac7-SR(BC2)]FS59-2-2-1-1-#/CML144(BC2)-9-5-2-1-3-1-#-4 | 2.4 | MR |
| 22 | [KIT/SNSYN[N3/TUX]]c1F1-##(GLS=2.5)-32-1-1-#/CML176BC1F1-12-1-3-2-1-#-1-B | 2.3 | MR |
| 23 | [KIT/SNSYN[N3/TUX]]c1F1-##(GLS=2.5)-32-1-1-#/CML176BC1F1-12-1-3-4-2-#-1-B | 1.5 | R |
| 24 | [POOL9Ac7-SR(BC2)]FS60-2-1-1-1-#/CML176(BC2)-1-3-2-3-3-#-2-B | 2.4 | MR |
| 25 | [POOL9Ac7-SR(BC2)]FS60-2-1-1-1-#/CML176(BC2)-1-3-2-3-4-#-1-B | 2.3 | MR |
| 26 | [POOL9Ac7-SR(BC2)]FS60-2-1-1-1-#/CML176(BC2)-1-3-2-3-5-#-1-B | 1.5 | R |
| 27 | [POOL9Ac7-SR(BC2)]FS60-2-3-1-3-1-#/CML176(BC2)-3-1-3-3-3-#-1-B | 2.3 | MR |
| 28 | SADVLA/CML154 BC2F54-4-1-B-#-4-# | 4.5 | S |
| 29 | SADVLA/CML154 BC2F41-1-12-B-#-#-# | 3.2 | MS |
| 30 | SADVLA/CML154 BC2F37-2-1-B-#-#-# | 2.7 | MR |
| 31 | P502 SR/CML384X176ÃÆâÃâââ¬Ãâæ..98-2-1-2 BC2F4-1-3-B-#-#-# | 4 | S |
| 32 | CML176 | 4.5 | S |
| 33 | CML491 | 3.5 | MS |
Table 2. Reaction of Maize (QPM) lines for Common leaf rust disease at Ambo, in 2015.
Among 32 normal maize lines, 22 (68.8%) lines showed resistant responses for Common leaf rust at Ambo, in 2015 (Table 3). Moderately resistant responses were recorded on seven normal maize lines. Out of 32 normal maize lines, moderately susceptible responses were recorded on B.T.Z.T.V.C -172-1-1-3-2-2-1-1-#-#-# and B.T.Z.T.R.L -83-B-1-3-1-3-1-3-#-#-# normal maize lines. Susceptible response was recorded on B.T.Z.T.V.C -171-1-1-2-3-2-#-#-#-#-# maize line at Ambo, in 2015.
| S.no | Maize lines | Severity (1-5 scale) | Line response |
|---|---|---|---|
| 1 | B.T.Z.T.V.C -43-B -2-2 -2-#-1-#-#-#-# | 1.3 | R |
| 2 | B.T.Z.T.V.C -43-B -2-2 -3-2-2-1-#-#-# | 1.2 | R |
| 3 | B.T.Z.T.R.L -83-B-1-3-1-3-1-1-#-#-# | 1.4 | R |
| 4 | B.T.Z.T.R.L -83-B-1-3-1-3-1-2-#-#-# | 1.5 | R |
| 5 | B.T.Z.T.R.L -83-B-1-3-1-3-1-3-#-#-# | 3.2 | MS |
| 6 | B.T.Z.T.V.C -43-B -2-3 -1-1-1-1-#-#-# | 2.2 | MR |
| 7 | B.T.Z.T.V.C -43-B -2-3 -1-3-2-#-#-#-# | 1.2 | R |
| 8 | B.T.Z.T.V.C -43-B -2-1 -1-2-2-#-#-#-# | 1.4 | R |
| 9 | B.T.Z.T.V.C -43-B -2-1 -1-3-1-1-#-#-# | 1.2 | R |
| 10 | B.T.Z.T.V.C -43-B-1-2 -1-1-1-#-#-#-# | 1.3 | R |
| 11 | B.T.Z.T.V.C -43-B-1-2 -1-2-1-#-#-#-# | 1.2 | R |
| 12 | B.T.Z.T.V.C -99-B-1-2 -1-1-1-#-#-#-# | 2.7 | MR |
| 13 | B.I.Z.T.V.C -83-B-3-3-3-1-#-#-#-# | 2.3 | MR |
| 14 | B.I.Z.T.V.C -85-B-2-1-3-1-#-#-#-# | 1.5 | R |
| 15 | B.I.Z.T.V.C -8-B-1-3-1-#-#-#-#-# | 1.6 | R |
| 16 | B.T.Z.T.V.C -266-B-1-2 -2-2-#-#-#-#-# | 1.4 | R |
| 17 | B.T.Z.T.V.C -138-B-2-3 -2-1-1-#-#-#-# | 2.3 | MR |
| 18 | B.T.Z.T.V.C -138-B-2-3 -3-1-2-1-#-#-# | 1.5 | R |
| 19 | B.I.Z.T.V.C -68-B-3 -2-2-1-#-#-#-# | 1.4 | R |
| 20 | B.T.Z.T.V.C -171-1-1-2-2-1-#-#-#-#-# | 1.3 | R |
| 21 | B.T.Z.T.V.C -171-1-1-2-2-2-#-#-#-#-# | 1.2 | R |
| 22 | B.T.Z.T.V.C -171-1-1-2-3-2-#-#-#-#-# | 4.0 | S |
| 23 | B.T.Z.T.V.C -172-1-1-3-1-1-2-#-#-#-# | 1.3 | R |
| 24 | B.T.Z.T.V.C -172-1-1-3-1-2-1-#-#-#-# | 1.5 | R |
| 25 | B.T.Z.T.V.C -172-1-1-3-1-2-2-#-#-#-# | 2.2 | MR |
| 26 | B.T.Z.T.V.C -172-1-1-3-2-2-1-1-#-#-# | 3.1 | MS |
| 27 | B.T.Z.T.V.C -172-1-1-3-2-2-1-2-#-#-# | 2.1 | MR |
| 28 | B.T.Z.T.V.C -172-1-1-3-3-1-1-#-#-#-# | 1.2 | R |
| 29 | SINT T.SR.B.T.Z.T.4P-1P-4P-1P-3P-6-1-1-1-#-#-# | 1.3 | R |
| 30 | SINT TSR.B.T.Z.T.19P-1P-1P-2P-1P-5-1-1-3-#-#-# | 2.6 | MR |
| 31 | B-62.5%9A TSR-19P-3P-1P-2P-1P-1P-2-1-#-2-#-#-# | 1.4 | R |
| 32 | B.T.Z.T.V.C.PR.93A 1-2P-1-2-1 -4-2-1-#-#-#-# | 1.7 | R |
Table 3. Reaction of maize (normal) lines for Common leaf rust disease at Ambo, in 2015.
Out of 20 QPM maize lines, 7 lines showed resistant (R) responses for Turcicum leaf blight (TLB) at Ambo, in 2016 (Table 4). Moderately resistant (MR) responses were recorded on 11 QPM maize lines, and only 2 lines showed moderately susceptible (MS) responses for this disease. Relatively higher Area under disease curve (AUDPC) and severity of Turcicum leaf blight was recorded on the normal maize lines than QPM maize lines at Ambo, in 2016 (Table 4).
| QPM maize line | Severity (1-5 scale) | AUDPC | Line responses | Normal maize line | Severity (1-5 scale) | AUDPC | Line responses |
|---|---|---|---|---|---|---|---|
| AMB15QTWP3-2 | 2.03c | 58.72bcde | MR | AMB15N-37LD-17 | 2.18b | 64.84efg | MR |
| AMB15QTWP3-4 | 2.05c | 67.79bcd | MR | AMB15N-37LD-24 | 1.04c | 7.69h | R |
| AMB15QTWP3-5 | 1.32d | 17.09f | R | AMB15N-37LD-26 | 3.25a | 136.11b | MS |
| AMB15QTWP3-8 | 1.08d | 14.30f | R | AMB15N-37LD-27 | 3.6a | 171.18ab | S |
| AMB15QTWP3-11 | 2.04c | 53.98bcde | MR | AMB15N-37LD-35 | 1.22c | 9.14gh | R |
| AMB15QTWP3-14 | 2.09bc | 65.07bcd | MR | AMB15N-37LD-36 | 3.25a | 135.06b | MS |
| AMB15QTWP3-15 | 1.28d | 15.44f | R | AMB15N-37LD-48 | 3.12a | 128.08bcd | MS |
| AMB15QTWP3-20 | 2.73ab | 112.49a | MR | AMB15N-37LD-49 | 1.25c | 15.59fgh | R |
| AMB15QTWP3-21 | 2.04c | 60.58bcd | MR | AMB15N-37LD-53 | 1.0c | 7.5h | R |
| AMB15QTWP3-22 | 3.05a | 117.34a | MS | AMB15N-23-39 | 2.17b | 73.28de | MR |
| AMB15QTWP3-26 | 2.04c | 41.61cdef | MR | AMB15N-21-1 | 1.01c | 7.56h | R |
| AMB15QTWP3-28 | 2.18bc | 67.19bcd | MR | AMB15N-21-5 | 3.77a | 196.48a | S |
| AMB15QTWP3-31 | 1.35d | 22.38ef | R | AMB15N-21-33 | 1.0c | 7.50h | R |
| AMB15QTWP3-32 | 1.26d | 16.91f | R | AMB15N-21-34 | 2.17b | 70.03ef | MR |
| AMB15QTWP3-33 | 2.1bc | 64.89bcd | MR | AMB15N-21-42 | 3.16a | 131.44bc | MS |
| AMB15QTWP3-36 | 1.37d | 22.39ef | R | AMB15KN20-8 | 2.26b | 75.30cde | MR |
| AMB15QTWP3-37 | 2.09bc | 72.67bc | MR | AMB15EN18-1 | 3.15a | 129.22bcd | MS |
| AMB15QTWP3-43 | 2.43abc | 85.05ab | MR | AMB15EN18-13 | 1.13c | 9.45gh | R |
| AMB15QTWP3-47 | 3.05a | 121.09a | MS | AMB15EN18-15 | 1.18c | 19.59efgh | R |
| AMB15QTWP3-48 | 1.36d | 31.17 | R | AMB15EN18-22 | 1.22c | 10.58gh | R |
| Mean | 1.95 | 56.41 | - | - | 2.11 | 70.28 | - |
| CV (%) | 15.86 | 31.22 | - | - | 16.67 | 38.40 | - |
| LSD (0.05) | 0.65 | 36.86 | - | - | 0.74 | 56.49 | - |
Table 4. Responses of maize lines for TLB disease at Ambo, in 2016.
Among 20 normal maize lines, 9 lines showed resistant responses for Turcicum Leaf Blight (TLB) at Ambo, in 2016 (Table 4). Moderately resistant responses were recorded on four normal maize lines. Moderately susceptible responses were recorded on five normal maize lines, and only 2 normal maize lines (AMB15N-37LD-27 and AMB15N-21-5) showed susceptible responses for TLB at Ambo, in 2016 (Table 4).
Among 20 QPM maize lines, five lines were showed resistant (R) responses for Common leaf rust (CLR) at Ambo, in 2016 (Table 5). Moderately resistant (MR) and moderately susceptible (MS) responses were recorded on ten and four QPM maize lines, respectively. Only one QPM maize line (AMB15QTWP3-32) showed susceptible (S) response for CLR disease at Ambo, in 2016 (Table 5).
| QPM maize line | Severity (1-5 scale) | AUDPC | Line responses | Normal maize line | Severity (1-5 scale) | AUDPC | Line responses |
|---|---|---|---|---|---|---|---|
| AMB15QTWP3-2 | 3.2ab | 129.7abcd | MS | AMB15N-37LD-17 | 1.01d | 7.53d | R |
| AMB15QTWP3-4 | 1.5f | 32.9gh | R | AMB15N-37LD-24 | 2.1c | 76.36bc | MR |
| AMB15QTWP3-5 | 1.2fg | 9.6h | R | AMB15N-37LD-26 | 1.0d | 7.5d | R |
| AMB15QTWP3-8 | 3.4a | 162.7ab | MS | AMB15N-37LD-27 | 1.0d | 7.5d | R |
| AMB15QTWP3-11 | 2.7cd | 104.7cde | MR | AMB15N-37LD-35 | 2.31bc | 87.58abc | MR |
| AMB15QTWP3-14 | 2.2e | 73.1efg | MR | AMB15N-37LD-36 | 2.5ab | 92.79ab | MR |
| AMB15QTWP3-15 | 2.7cd | 103.3cde | MR | AMB15N-37LD-48 | 2.38abc | 80.08abc | MR |
| AMB15QTWP3-20 | 3.3a | 148.7abc | MS | AMB15N-37LD-49 | 1.03d | 7.75d | R |
| AMB15QTWP3-21 | 2.6cd | 99.8de | MR | AMB15N-37LD-53 | 1.05d | 7.81d | R |
| AMB15QTWP3-22 | 2.4cde | 83.9def | MR | AMB15N-23-39 | 2.18bc | 66.60c | MR |
| AMB15QTWP3-26 | 1g | 41.3fgh | R | AMB15N-21-1 | 1.0d | 7.5d | R |
| AMB15QTWP3-28 | 2.8bc | 118.5bcde | MR | AMB15N-21-5 | 1.0d | 7.5d | R |
| AMB15QTWP3-31 | 1g | 7.5h | R | AMB15N-21-33 | 1.0d | 7.5d | R |
| AMB15QTWP3-32 | 1g | 7.5h | R | AMB15N-21-34 | 1.0d | 7.5d | R |
| AMB15QTWP3-33 | 3.6a | 171.03a | S | AMB15N-21-42 | 2.7a | 107.72a | MR |
| AMB15QTWP3-36 | 2.1e | 78.7efg | MR | AMB15KN20-8 | 2.16bc | 64.58c | MR |
| AMB15QTWP3-37 | 3.4a | 154.4ab | MS | AMB15EN18-1 | 2.18bc | 79.73bc | MR |
| AMB15QTWP3-43 | 2.4de | 84.6def | MR | AMB15EN18-13 | 2.12bc | 69.27bc | MR |
| AMB15QTWP3-47 | 2.1e | 78efg | MR | AMB15EN18-15 | 1.03d | 7.73d | R |
| AMB15QTWP3-48 | 3.5a | 177.4a | MR | AMB15EN18-22 | 1.0d | 7.5d | R |
| Mean | 2.4 | 93.4 | - | - | 1.59 | 40.80 | - |
| CV (%) | 8.1 | 24.9 | - | - | 11.49 | 29.95 | - |
| LSD (0.05) | 0.41 | 48.6 | - | - | 0.38 | 25.57 | - |
Table 5. Responses of maize lines for CLR disease at Ambo, in 2016.
Out of 20 normal maize lines, eleven lines showed resistant responses for CLR disease at Ambo, in 2016 (Table 5). Moderately resistant responses were recorded on nine normal maize lines. Relatively higher AUDPC and severity of CLR was recorded on QPM maize lines than normal maize lines at Ambo, in 2016 (Table 5).
Turcicum leaf blight (TLB) and Common leaf rust (CLR) is among the major foliar diseases of maize in Ethiopia. Screening was done at Ambo (TLB and CLR) and Bako (TLB only) for two consecutive years (2015 and 2016 growing seasons) in order to know the responses of maize lines (QPM and Normal maize lines) for two diseases. This was done on 73 maize lines (53 QPM and 20 Normal) for TLB, and on 105 maize lines (53 QPM and 52 normal) for CLR.
Among 33 tested quality protein maize (QPM) lines, 12 (36.3%) lines were showed resistant responses for Turcicum leaf blight in 2015, only at Ambo. From 33 maize lines, 14 (42.4%) lines showed resistant responses both at Ambo and Bako, in the same growing season. Moderately responses were recorded at Ambo and Bako on seven and eighteen maize lines in 2015, respectively. Only one QPM maize line showed susceptible response at Bako experimental site. Out of 40 maize lines (20 QPM, and 20 Non-QPM), resistant responses were recorded on 7 QPM and 9 normal maize lines for TLB disease in 2016, at Ambo. Two normal maize lines were showed susceptible responses for TLB disease at Ambo, in the same growing season, 2016.
Among 33 QPM maize lines, 6 (18.2%) line showed resistant responses for CLR disease in 2015 at Ambo. Susceptible responses were recorded on 3 QPM maize lines for CLR disease at Ambo in the same growing season. Out of 32 normal maize lines, 22 (68.7%) lines showed resistant responses; and susceptible response was recorded on 1 line in 2015, at Ambo. Among 40 maize lines (20 QPM, and 20 Non-QPM), resistant responses were recorded on 5 QPM and 11 normal maize lines in 2016, at Ambo. One QPM-maize line was showed susceptible response for CLR disease at Ambo, in the same growing season.
In general, higher mean severity and mean AUDPC of CLR was recorded on QPM than normal maize lines whereas higher mean severity and mean AUDPC of TLB was recorded on normal maize lines than QPM maize lines. Therefore, attention should be given for both diseases during screening in order to develop resistant maize varieties for both maize types. Those selected resistance maize lines from this screening should be used in breeding program and finding of resistant maize lines for both diseases will be continued using modern screening tools as well as techniques in addition to this conventional method.
I would like to thank Ethiopian Institute of Agricultural research and Ambo Plant Protection Research Center for providing support in different aspects during the research time.