ISSN:2321-6212
ISSN:2321-6212
1Department of Material Science Engineering, Sahand University of Technology, Tabriz, Iran
2School of Materials Science and Engineering, Shiraz University, Shi raz, Iran
3Institute of Nano Science and Nano Technology, University of Kashan, Kashan, Iran
Received Date: 04/01/2021; Accepted Date: 30/09/2021; Published Date: 07/10/2021
Visit for more related articles at Research & Reviews: Journal of Material Sciences
Electrophoretic deposition is a process mainly depended on colloidal stability of deposition suspension. In this work, colloidal stability of submicron beta-sialon in ethanol was studied. Zeta potential and viscosity of beta-sialon powder suspension as a function of pH were measured in this solvent. The effect of addition of PVA polymer to the suspension was also investigated. Viscosity was measured as an indicator of dispersion quality of solvent. The effect of polymer addition to the beta-sialon suspension on zeta potential was also determined as a function of pH. Lastly, Electrophoretic Deposition (EPD) was carried out using the identified optimum suspension conditions. The optimum working condition was also determined
Stable condition, Electrophoretic Deposition (EPD), Suspension and working condition, Beta-Sialon.
Sialons are ceramics based silicon nitride. This family of material is solid solutions of 3 i3N4.These ceramics are excellent for their excellent mechanical properties such as high strength, wear resistance, high hardness and high thermal shock resistance[1-4]. Sialon ceramics have found application in handling of molten non-ferrous metals particularly aluminium and its alloys. Crucibles, ladles, injector and degassing equipment [5].for nonferrous metals, burner and immersion heater tubes [6].metal feed tubes for aluminium die casting, thermocouple protection tubes[7] and cutting tools [8-10]. Which are produced by Sialon have a good performance. The electrophoretic deposition (EPD) process is based on the movement of the charged particles in the suspension by applying an external electric field [11]. This process has several advantages such as: simple equipment requirement, short depositing time and little restriction of substrate shape, making it become a cost-effective and versatile process [12].We have proposed that EPD is a promising technique to prepare a thickness-controlled Sialon layer on a substrate [13].
Experimental procedure
Beta-sialon powder with average size of 0.5 μm was prepared by ball milling of Beta-sialon powder with average size of 1.0 to 2.0 ��m (AG material Inc, Taiwan) for 5 h. This powder was used for preparing colloids with 4 g beta-sialon in 100 ml ethanol (99.8%) at various pH for zeta potential and viscosity measurements. The pH values were adjusted by using HNO3 and NH3 for acidic and alkaline ranges, respectively. Each colloid sample was also ultrasonically dispersed for 30 min to ensure a good dispersion of the particles. Viscosity of the suspension was measured using a viscometer (Brookfield Viscometer RVDV-ll-Pro, USA) at a shear rate of 1800 S-1. The zeta potential and the electro mobility of the suspension at different pH were measured using a zeta potential analyser (SZ-100 HORIBA Scientific Japan). During EPD, colloids of concentration 4 g per 100 ml of ethanol (99.8%) were prepared at pH=4 for suspensions with and without PVA addition. Constant voltage of 30 V/cm was applied during the process. The electrodes used were stainless steel for anode and cathode. The deposition mass was quantified by measuring the weight gained after deposition. Cross-sectional deposit examination for thickness measurement was carried out using Scanning Electron Microscope (model S-3700N, Hitachi, Japan) and the deposition density was then calculated.
Stability of suspension
The zeta potential is a key indicator of the stability of colloidal dispersions. Figure 1 shows the zeta potential as a function of pH. Generally high absolute zeta potential values indicate a high stability of suspension and high particle dispersion. As shown in Figure 1, the isoelectric point (IEP) of beta sialon colloids is at pH=6. At this point, there is no surface charge and so that, the zeta potential is zero. The suspension with pH=6 (ζ=0) tend to agglomerate because the electrostatic interaction is minimum at this point and there is no surface charge in this point. Figure 2 shows the viscosity of beta sialon as a function of pH. It is noted that maximum viscosity occurred at pH=6. This maximum viscosity indicates a high degree of particle flocculation in the colloid, which is consistent with the zeta potential measurement shown in Figure 1.
One of the important factors in the stability of the suspension is the particle size. In the present case, the mean particle size was measured to be 0.5 μm. It is well accepted that particles larger than 1.0 μm do not provide good colloid stability as the sedimentation of the particles occurs very quickly.
The effect of PVA on stability of suspension
Development of electric double layer in a non- aqueous medium such as ethanol is difficult, so addition of branched polymers such as PVA increases the stability of the suspension. In such situations, the stability of the colloid is usually enhanced through electrostatic interactions between the particles that resulted from polymer addition to the suspension. The polymer chains adsorb onto the surface of the ceramic particles. A repulsive steric interaction will occur when these particles with polymer chains on their surface, come close to each other. Lewis [14] used the Flory-Huggins parameter, χ, to represent the quality of the polymeric solution.
It is noted that the quality of solvent, α, cab be simply represented through viscosity measurement. The relationship can be written as: Development of electric double layer in a non- aqueous medium such as ethanol is difficult, so addition of branched polymers such as PVA increases the stability of the suspension.
In such situations, the stability of the colloid is usually enhanced through electrostatic interactions between the particles that resulted from polymer addition to the suspension. The polymer chains adsorb onto the surface of the ceramic particles. A repulsive steric interaction will occur when these particles with polymer chains on their surface, come close to each other. Lewis [14] used the Flory-Huggins parameter, χ, to represent the quality of the polymeric solution. It is noted that the quality of solvent,α , cab be simply represented through viscosity measurement. The relationship can be written as:
η = BMα
Which M is the molecular weight of added polymer, B is constant and α can vary between 0.5 and 0.8.
By rewriting Eq. (1), we obtain 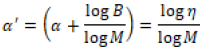
Which η is viscosity, α^' is the solvent quality parameter as a function of viscosity. As B and M are constant for an additive, α^’ represents the dispersing ability of the solvent. A higher α^' value will indicate a solvent with most polymer chains in suitable situation, hence providing a solvent with higher dispersing ability Figure 3. shows the result of computed solvent quality parameter α^' at various pH for different amount of polymer addition, namely 6 and 12 mg. As shown in the Figure 3. the amount of quality parameter α^' varies with ph. At pH below 6, a relatively higher dispersing quality is measured. It is observed that the suspension with 12 mg PVA addition shows a better dispersion ability that the suspension with 6 mg PVA addition. It is concluded that suspension with pH lower that 6, has the highest stability and is suitable for EPD.
Figure 4. shows the influence of polymer addition on zeta potential of the beta sialon suspension as a function of pH. It can be seen that in pH range 3–8, the addition of PVA to provide steric stabilization has shifted the IEP to a lower pH value. It is also noted that when polymers are adsorbed onto the particle surface, changes in the ion distribution in the diffuse double layer will affect the absolute zeta potential.
Electrophoretic deposition of beta sialon
Electrophoretic deposition is a process that mainly depended on stability of suspension. In fact the optimum parameters of suspension are very important for obtaining a uniform coating. After determining the optimal condition of the suspension, including pH and the amount of polymer additive, electrophoretic deposition was performed on the stainless steel substrate. A constant voltage of 30 Volt/cm was applied for 60 seconds. The deposition thicknesses of different suspensions were measured under SEM under a fixed deposition area. The deposition masses were measured and the green packing densities for the different suspensions, namely, no PVA addition, 6 mg PVA addition and 12 mg PVA addition, were determined to be 52%, 65% and 67%, respectively. The higher green density for suspension with 12 mg PVA, have shown that this suspension was well- dispersed. It is also noted that there was difference in green densities between suspension with and without PVA.As a result, it shows that addition of PVA increases the dispersion ability of suspension.
The stability of beta sialon suspension and the effect of PVA addition on the stability of beta sialon suspension were separately examined in this work. Variation in zeta potential and viscosity relative to pH were measured. By comparing variation in zeta potential and viscosity for beta sialon suspension, it has been showed that there is an optimum range of pH values. For optimal range, electrophoretic deposition is well performed on the substrate. It is also shown that PVA addition can affect the zeta potential value at high ionic concentrations. EPD experiments have demonstrated that the suspensions prepared at the identified optimum conditions were well-dispersed and resulted in the formation of high green density deposits.