ISSN: 2319-9873
ISSN: 2319-9873
Department of Chemistry, Laxminarayan Institute of Technology, RTM Nagpur University, Nagpur 440010, India
Received: 25/08/2013 Accepted: 01/10/2013
Visit for more related articles at Research & Reviews: Journal of Engineering and Technology
Terpolymer resin (4-ASAOF-III) has been synthesized by the condensation polymerization of 4-Aminosalicylic acid (4-ASA) and Oxamide (O) with Formaldehyde (F) in the presence of 2M HCl as a catalyst using 3:1:4 molar ratios of reacting monomers. The empirical formula and empirical weight of the resin were determined by elemental analysis. The structure of the resin was characterized by various spectral techniques like infra-red (FTIR) and nuclear magnetic resonance (1H and 13C-NMR) spectroscopy. The morphological feature of the 4-ASAOF-III terpolymer resin was established by Scanning electron microscopy (SEM). Thermal study of the resin was carried out to determine its mode of decomposition and relative thermal stability. The Freeman–Carroll, Sharp Wentworth, Friedman and Change technique have been used in the present investigation to calculate thermal activation energy (Ea), order of reaction (n) and frequency factor (z).
Terpolymer, Characterization, Thermo gravimetric analysis, Activation energy
Three component reactions are the subject of extensive interest amongst the polymer scientists. Various method of preparation has been investigated and applied so far, but condensation polymerization of reacting monomers is the most efficient method for the synthesis of terpolymer resins [1-3]. In order to predict the stability, kinetics and thermal degradation behaviour; thermogravimetric study is necessary which assist to investigate its high performance utility in domestic as well as industrial sector.
The terpolymers offered novelty and versatility; hence they occupy the pivotal position in the field of material science. The progress in the field of terpolymer has been extensively rapid, as they are useful in packaging, adhesive, coating in electrical sensors and organometalic semiconductors [4-6]. Polymer additives improve manufacture process and product quality. It can form continuous phase of coating with no deleterious effects on coatings, and having better thermal stability [7-9].
Appreciable research work has been carried out on synthesis and characterization of terpolymers in our laboratory [10-12]. Shah et al have reported the chelating ability of resin synthesized by a microwave irradiation technique involving salicylic acid and formaldehyde with resorcinol [13]. Patel et al [14] have prepared the terpolymer of salicylic acid/p-hydroxybenzoic acid and thiourea with trioxane in presence of acid catalyst with different molar proportion of monomers. Jadhav et al have reported the synthesis, characterization and thermal degradation kinetics of copolymers derived from 2,2’-dihydroxybiphenyl- formaldehyde [15] copolymer and 2,2’-dihydroxybiphenyl, urea, formaldehyde [16] terpolymer. Pradip Paik and Kamal K. Kar [17] studied the kinetics of thermal degradation and estimation of lifetime for polypropylene particles and its effect on particle size, involving the use of single as well as multiple heating rate techniques. However, the literature studies have revealed that no terpolymer has been synthesized using the monomer, 4-aminosalysilic acid, oxamide and formaldehyde.
In this article, we present the synthesis and characterization of terpolymer (4-ASAOF-III) derived from 4-aminosalicylic acid and oxamide using the linkage of formaldehyde. The newly synthesized terpolymer has been characterized by elemental analysis and spectral methods. Thermal degradation behaviour was studied by TGA under nonisothermal conditions. Certain generalizations are made regarding the kinetic parameters computed by using Freeman-Carroll, Sharp-Wentworth, Friedman and Chang technique.
Materials
4-Aminosalicylic acid and oxamide is of analytical grade purity which is purchased from Acros Chemicals, Belgium and formaldehyde (37%) was purchased from S. D. Fine Chemicals, India. All the used solvents like N, N-dimethylformamide, dimethyl sulphoxide, tetrahydrofuran, acetone, diethyl ether were procured from Merck, India.
Synthesis
The terpolymer 4-ASAOF-III was prepared by condensing 4-aminosalicylic acid (4.59g, 0.3mol) and oxamide (0.88 g, 0.1mol) with formaldehyde (15 ml, 0.4mol) in presence of 2 M HCl as a catalyst in the molar proportion of 3:1:4 at 130 °C in an oil bath for 5 hrs. The temperature of electrically heated oil bath was controlled with the help of a dimmerstat. The dark reddish brown resinous solid product was immediately removed, filtered and repeatedly washed with cold-distilled water, dried in air and powdered with the help of an agated mortar and pestle. The product obtained was extracted with diethyl ether to remove excess of oxamide formaldehyde copolymer which might be present along with 4-ASAOF-III terpolymer. Dried resin sample was dissolved in 8 % NaOH and regenerated using 1:1 HCl/water (v/v) with constant stirring and filtered. This process was repeated twice. Resulting copolymer sample was washed with boiling water and dried in a vacuum at room temperature. Purified copolymer resin was finely ground to pass through 300-mesh size sieve and kept in a vacuum over silica gel [18]. The chemical reaction of above synthesis is given in figure.1.
Spectral and thermal studies
Terpolymer was subject to elemental analysis for carbon, hydrogen and nitrogen on Perkin Elmer 2400 Elemental Analyser. Infrared spectram was recorded in Frontier-Transform Infra Red spectrophotometer, Shimadzu in the range of 4000-500 cm-1. 1H-NMR studies were performed in dimethylsulphoxide as solvent on Bruker Advance-II 400 MHz proton NMR spectrophotometer and 13C-NMR spectrum was also recorded using Bruker 100 MHz. All the analytical and spectral studies for newly synthesized terpolymers were carried out at Sophisticated Analytical Instrumentation Facility (SAIF) Punjab University, Chandigarh. The non-isothermal thermogravimetric analysis of newly prepared terpolymers has been carried out using Perkin Elmer, Pyris1 Thermogravimetric Analyzer, in air atmosphere with a heating rate 10 °C.min-1 in the temperature range 40-1000 °C. TGA was carried out at VNIT, Nagpur.
Elemental analysis
The yield of resin was found to be 87%. Composition of terpolymer obtained on the basis of elemental analysis data and was found to be in good correlation to that of calculated values as given in table. 1.
FT-IR spectra
The FT-IR spectrum of 4-ASAOF-III terpolymer is represented in Figure 2 and the data is reported in Table 2. Broad band appeared at 3271.27 cm-1, which may be assigned to the stretching vibration of the phenolic -OH groups exhibiting intermolecular hydrogen bonding [19,20]. The presence of a weak peak at 2924.09 cm-1 describes the –NH- in 4-aminosalicylic acid and oxamide moiety which might be present in terpolymeric chain [21]. A sharp and weak peak obtained at 2854.05 cm-1, indicates the presence of stretching vibrations of methylene group (–CH2-) in the terpolymer chain21. A medium band, displayed between 1199.72 cm-1 and 1346.31 may be due to stretching vibration of >C=O of phenol and carboxylic acid respectively. The band appeared between 1469.0 to1540.0 cm-1 is due to the presence of >C=C< in aromatic ring in terpolymer. The broad band appearing in the spectrum at 3480.1 cm-1 is assigned to the hydroxyl group of –COOH present in the aromatic ring and involves intramolecular hydrogen bonding with the –NH of Ar-NH2 [22]. This band seems to be merged with the band arising from –NH stretching vibrations of the Ar-NH2 group, and this is further confirmed by the –NH bending vibrations appearing at 1591.27 cm-1 [23]. The presence of pentasubstituted of aromatic ring21 is recognized from the weak band appearing at 800.46 and 1006.0 cm-1.
1H- NMR spectra
1H-NMR spectral data is given in Table 3 and spectrum is presented in Figure 3. Spectra reveal different patterns of peaks, since each of them possesses a set of protons having different proton environment. A significant downfield in chemical shift of proton of phenolic -OH group, observed at δ 5.14 ppm, is due to intermediate proton exchange reaction of phenolic –OH group [24,25]. A weak singlet is observed at δ 6.0 to 7.8 ppm may be due to the presence of aromatic proton in 4-aminosalicylic acid, the singlet observed in the region δ 4.32 is attributed to methylenic protons of Ar-CH2–NH moiety and another singlet observed at δ 8.10 ppm due to the presence of –NH- proton of -CH2–NH-CO moiety. A broad singlet observed at δ 4.10 ppm may be assigned to Ar -NH2- moiety. Singlet observed at δ 10.98 ppm may be assigned to acidic proton of Ar –COOH moiety [24,25]. A singlet observed in the region of δ 3.05 and δ 2.51 ppm assigned due to Ar-CH2-Ar and Ar-CH2 respectively.
13C-NMR spectra
The 13C-NMR spectrum of 4-ASAOF-III terpolymer is shown in Figure 4 and observed chemical shift assigned on the basis of the literature [21,24]. The C1 to C6 of the first aromatic ring shows the peaks at 151.9, 110.3, 135.7, 135.3, 127.1 and 137.7 ppm respectively and the peaks appeared at 39.8 ppm is assigned to the methylene carbon of Ar-CH2-NH linkage [21]. The peaks appeared at 172.4 ppm is due to the carbon of the carboxylic acid group and peaks appeared at 30.1 ppm is due to -CH2 carbon of Ar-CH2-Ar linkage. The peaks at 152.5, 112.6, 145.5, 135.5, 132.5 and 111.1 ppm with respect to C1 to C6 of second aromatic ring and peaks at 152.7, 113.7, 116.6, 138.2, 145.7 and 114.6 ppm with respect to C1 to C6 of third aromatic ring of terpolymer resin [26].
SEM analysis
The typical microphotograph at 1,500 magnification from SEM of 4-ASAOF-III is shown in Figure 5. The SEM image shows the surface future of the sample. The image of the 4-ASAOF-III is clearly indicative of a loosely close packed structure with high porosity or voids. The voids presents in the terpolymer ligands may be responsible for the swelling behavior and reactivity of active sites buried in the polymer matrix. The image also showed a transitions state between the amorphous and crystalline states. However, more predominantly the terpolymer is amorphous, because of the polycondensation reaction [27].
Thermogravimetric analysis
Thermal degradation behaviour and kinetic data of terpolymer is recorded in Table 4 and thermogram is shown in Figure 6. The resin 4-ASAOF-III exhibit three stages of decomposition in temperature range 40-1000°C, after loss of water molecule. When temperature was raised to 120 °C, it showed the weight loss about 5.69 % and is corresponding to the moisture entrapped in molecule or water of crystallization associated with this copolymer [28,29]. This is in well agreement with the weight loss calculated theoretically which is about 5.66 %. The First step is slow decomposition in temperature range 130-350 °C, corresponding to 42.72 % mass loss which may be attributed to loss of three hydroxyl, three amine and carboxylic acid groups against calculated value of 41.55 % loss present per repeat unit of polymer. Second step of decomposition starts from temperature 350-700 °C, corresponding to 86.39% loss of aromatic nucleus with methylene linkage against calculated 87.04% and third step decomposition start from 700-1000°C which corresponds to loss of 100% of oxamide moiety against calculated is found to be 100%. Consequently no residue may be assigned after complete degradation.
Thermogram expresses the dependence of change in mass on the temperature which gives information about sample composition, product formed after heating and kinetic parameters. Kinetics parameters have been determined using Friedman [30,31], Chang [32], Sharp-Wentworth [33], and Freeman-Carroll [34] techniques as follows
Friedman technique
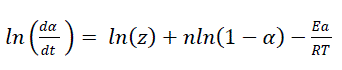 (1)
(1)
Where, α is the conversion at time t; R is the gas constant (8.314 J/mol/K) and T is the absolute temperature (K). From the slope of the linear plot of ln(1-α) vs. 1/T, n can be obtained. The plot of ln(dα/dt) vs. 1/T should be linear with the slope Ea/R, from which Ea can be obtained (Figure 7).
Chang technique:
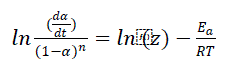 (2)
(2)
Figure 7 has shown Chang method (2) gives plots between [ln(dα/dt)/(1-α)n] vs. (1/T) which is used to calculate Ea and z of respective degradation reaction for best fitted value of n (from Friedman equation), which corresponds to correct reaction order for thermal decomposition.
Sharp-Wentworth technique:
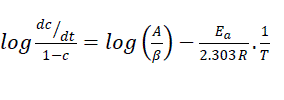 (3)
(3)
Where, dc/dt = rate of change of fraction of weight with change in temperature; β is linear heating rate. The linear plot of 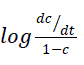 versus1/T is obtained whose slope gives the value of Ea and A may be evaluated from the intercept (Figure 7). The linear relationship confirmed that the assumed order n = 1 is correct.
versus1/T is obtained whose slope gives the value of Ea and A may be evaluated from the intercept (Figure 7). The linear relationship confirmed that the assumed order n = 1 is correct.
Freeman and Carroll technique:
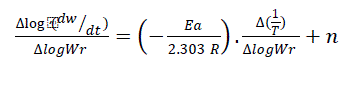 (4)
(4)
Where, dw/dt = rate of change of weight with time. Wr = Wc – W; Wc = Weight loss at the completion of reaction; W = Total weight loss upto time. Ea = Energy of activation; n = order of reaction. The Δlog (dw/dt) and Δlog Wr values are taken at regular intervals of 1/T. In this case 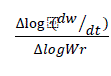 vs
vs 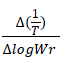 gives a straight line. The slope and intercept are equal to - (Ea/R) and n, respectively. (Figure 7)
gives a straight line. The slope and intercept are equal to - (Ea/R) and n, respectively. (Figure 7)
A plot of percentage mass loss vs temperature is shown in Figure 6 for a representative 4-ASAOF-III terpolymer. From the TG curves, the thermoanalytical data in Table 5. This kinetic analysis should be a starting point to obtain the useful information on the behavior of samples. Fairly comparable results in the kinetic parameters i.e. Ea, n and z are obtained by Friedman and Chang may be due to analogy in mathematical model. Also fairly similar results with slight variations obtained by Sharp-Wentworth and Freeman-Carroll methods, Fairly good straight line plots are obtained using two methods. This is expected since the decomposition of terpolymer is known not to be obeying first order kinetics perfectly [35].
From the above discussion, it is therefore concluded that for each technique, the values of kinetic parameters depend on calculation technique used. Total calculations obtained from different kinetic models demonstrated that the numerical value of kinetic parameters depends on the mathematical model used to analyze the experimental data and level of degradation [17]. Due to complex phenomena of terpolymer degradation process in non-isothermal thermogravimetry, the computed kinetic parameters are in fact only parameters of given mathematical equation which has the form of kinetic rate equation and which is used to fit the weight loss curves accompanying the thermal degradation of polymers in non-isothermal conditions. Low values of frequency factor revealed that decomposition reaction of terpolymer may be slow and no other possible reason can be given [36,37]. As a consequence these kinetic parameters are fictive from the point of view of chemical kinetics.
1. Synthesis of targeted terpolymer (4-ASAOF-III) has been confirmed which is supported by the results obtained by the elemental analysis and spectral data.
2. Presence of spectral peaks of Methylene Bridge in the spectral data confirms the formation of terpolymer resin.
3. The activation energy values calculated from Freeman-Carroll and Sharp-Wentworth method are in well agreement with each other, indicating common reaction mode.
4. Friedman, Chang methods show nearly similar values of kinetic parameters may be due to resemblance in mathematical model
The authors wish to express their sincere thanks to University Grand Commission, New Delhi. Authors would also like to thank SAIF, Punjab University, Chandigarh for carrying out spectral analysis and also to VNIT, Nagpur for providing thermogravimetric facility.