e-ISSN: 2319-9849
e-ISSN: 2319-9849
Ashraf Duzan1,2,3*, Mufeed M. Basti2, Waldemar Debinski4
1 Department of Pharmacy, Wingate University, Wingate, USA
2 Department of Applied Science and Technology, North Carolina State University of Agriculture and Technology, Greensboro, USA
3 Department of Pharmaceutical Sciences, Nova Southeastern University, Fort Lauderdale, USA
4 Department of Biochemistry, Wake Forest University, Winston-Salem, USA
Received: 21-Jul-2023, Manuscript No. JCHEM-23-107559; Editor assigned: 24-Jul-2023, PreQC No. JCHEM-23-107559(PQ); Reviewed: 07-Aug-2023, QC No. JCHEM-23-107559; Revised: 14-Aug-2023, Manuscript No. JCHEM-23-107559(R); Published: 23-Aug-2023, DOI: 10.4172/2319-9849.12.3.001
Citation: Duzan A, et al. Unveiling the Potential of Cannabis Extracts: Chemical Composition and Pharmacological Insights for Glioblastoma Therapy. RRJ Chemist. 2023;12:001.
Copyright: © 2023 Duzan A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Research & Reviews: Journal of Chemistry
Glioblastoma Multiforme (GBM) is an aggressive and metastatic brain tumor with a low success rate in treatment, particularly in immune checkpoint-active tumors, resulting in less than three percent of patients surviving beyond five years. Targeted treatments specifically designed for GBM are urgently needed. Our studies have examined the effects of Chemovar Specific Cannabis Extractions (CSCEs), which are cannabis extracts obtained using polar solvents and analysed using Liquid column Chromatography combined with Mass Spectrometry (LC/MS). However, the complex nature of cannabis compounds has hindered the personalization of standard cannabis medicines for GBM due to the unknown synergistic effects of multiple compounds. To address this challenge, our study focuses on exposing U570 cells (a type of brain tumor cell) to chemovar fractions extracted using polar (upper layer) and nonpolar (lower layer) solvents. This approach enables the isolation of a broader spectrum of constituents present in the cannabis extract. By utilizing LC/MS in conjunction with Nuclear Magnetic Resonance (NMR), we have identified and quantified 11 cannabinoid compounds present in the polar CSCE that individually exhibit significant efficacy in inducing cell death in GBM tumor cells. Conversely, the polar fraction in our experiment did not demonstrate efficacy against U570 cells. The ability to quantify individual compounds within a cannabis extract that selectively induce cell death in brain tumors holds promise for guiding future research and facilitating the development of a standardized CSCE for GBM therapy.
Cannabinoids; Liquid chromatography; Mass spectrometry; Glioblastoma multiforme; Nuclear magnetic resonance; Anticancer activity
Gliomas, a type of brain tumor, pose a significant challenge in the field of oncology. Among them, glioblastoma (GBM) stands out as an especially aggressive and lethal form, classified as a Stage IV glioma according to the World Health Organization (WHO) [1,2]. Recent advancements in understanding the molecular aspects of Central Nervous System (CNS) tumors have led to the incorporation of molecular parameters in their classification, with substantial revisions made by the WHO in 2021 [1,2]. The Cancer Stem Cell (CSC) theory is gaining momentum in the scientific community, proposing that glioblastomas originate from glial cells undergoing abnormal differentiation, leading to the formation of cancer stem cells. These CSCs are believed to drive tumor initiation and progression [3,4]. However, unraveling the precise origins of individual GBM tumors remains an ongoing research endeavour, underscoring the need for further investigation.
In the clinical setting, standardized treatments have shown promising results in improving survival rates for GBM patients. The current standard of care involves surgical resection, radiation therapy, and chemotherapy, particularly using daily temozolomide (TMZ) [5,6]. This combined approach has demonstrated median survival rates of up to 20 months. However, treatment effectiveness greatly depends on the genetic makeup of each tumor, as specific mutations can confer resistance to therapy. Notably, the absence of O6-Methylguanine-DNA Methyltransferase (MGMT) promoter methylation is a critical determinant of TMZ efficacy, with GBM lacking this methylation showing resistance to the drug [7-9]. Considering these challenges, novel therapeutic strategies are being explored, and cannabis metabolites have emerged as a promising avenue. These metabolites have demonstrated the ability to target several druggable mechanisms observed in genotype specific GBM. Despite these promising findings, additional research is still needed to establish a replicable and effective therapeutic approach utilizing cannabis metabolites [10].
Cannabis sativa L. encompasses a unique system of non-polar metabolites [11,12]. CBGA serves as a precursor for naturally occurring phytocannabinoids with a five-carbon chain, and its two primary metabolites are Tetrahydrocannabinolic Acid (THCA) and Cannabidiolic Acid (CBDA) [13]. Non-polar constituents of cannabis include 180 known phytocannabinoids, 111 terpenes comprising either ten or fifteen carbons, and 121 terpenoids [14-16]. Fractions containing Δ-9-THCA, CBG, and CBC derived from a crude CSCE produced with polar solvents have induced ~ 90% cell death in the A172 GBM cell line after 48 hours of exposure [10]. Compliant extraction techniques and LC/MS quantification can standardize personalized CSCEs [10,16,17] and target specific GBM-related mechanisms, such as promoting 2-AG, ERK via CBrs, or PPARs [18-20], regulating AKT and cAMP through allosteric modulation [21-23], desensitizing Transient Receptor Potential channels (TRPs) [24], inhibiting GPR55 [25,26], or reducing Prostaglandin E2 (PGE2) via COX-2 [27-29].
Δ-9-tetrahydrocannabinol, a decarboxylated cannabinoid, activates the G protein-coupled receptors CB1r and CB2r [30,31]. It causes significant intoxication but also promotes angiogenesis and maintains ceramide homeostasis [32] and partially inhibits autotoxin [33]. In contrast, Cannabidiol (CBD) reduces calcium current by antagonizing GPR55 and negatively binds to the allosteric pocket in CB1r and CB2r with low efficacy [34,35]. Notably, when co-administered with an equal ratio of THC, CBD did not affect the ERK or P13K pathways in the ventral hippocampi, in contrast to each cannabinoid alone [22]. CBD has been shown to induce autophagy in a neuroblastoma cell line dependent on ERK1/2 and PI3K/AKT activation [36]. Therefore, the development of drug treatments must consider the biased conformational changes downstream of CB1r during the treatment of GBM with CSCEs [10,18,22].
CBG weakly binds to CB2r, while Cannabichromene (CBC) and Beta-Caryophyllene (BCP) act as selective CB2r agonists [37,38]. Glial cells in the cerebellum express CB2r following oxidative stress [39-42]. Cannabinoids downregulate calcium and sodium currents via TRP desensitization or inactivation, and CBD induces cell death in glioblastoma by dephosphorylating TRPV1 and inhibiting GPR55 [26]. TPRV1 strongly interacts with PGE2 [43], which is metabolized by COX-2. CBDA, THCA, and CBG inhibit COX-2 with significant efficacy, potentially mediating GBM proliferation by targeting arachidonic acid metabolism to PGE2 [27,29]. Clinical COX-2 inhibitors previously failed due to widespread adverse reactions.
Cannabinoid formulations also impact enzymes responsible for endocannabinoid metabolism and catabolism. CBD inhibits FAAH, which metabolizes N-arachidonoylethanolamine (AEA/anandamide) in the post-synaptic cleft of CB1r [24,44]. However, glioblastoma does not migrate away from the brain, where 2-AG levels are approximately 170-fold higher than anandamide [45]. Whole-plant extracts containing THCA, CBGA, and CBG, but not cannabinoid isolates, inhibit the serine hydrolase known as MAGl, which metabolizes 2-AG in the pre-synaptic cleft [24]. THC purified from cannabis extracts did not enhance the cytotoxicity of CBG against glioblastoma, whereas CBG induces cell death in cancer stem cells, targeting treatment-resistant tumors [26,46].
An earlier clinical trial using cannabis containing THC, with TMZ as a control, exhibited cytotoxic effects [47]. To investigate the toxicity effect of bioactive constituents in a whole-plant extract and identified them with polar extraction and quantified with LC/MS coupled with NMR could replicate positive results against GBM.
Chemicals and reagents
Mass spectrometry-grade formic acid, methanol, hexane, and acetonitrile (methyl cyanide) were purchased from fisher scientific (Waltham, MA). Optima-grade water was used for LC-MS analysis. The acquity Ultra-high-Performance Liquid Chromatography (UPLC) BEH C18 analytical column and vanguard pre-column for chromatography were obtained from waters Corp., Waltham, MA. Cannabis dry flower samples were obtained from local hemp stores in Wingate, North Carolina, USA. These products were stored at -20ºC until further analysis. Deuterated chloroform (CDCl3) with 1% TSP (trimethylsilyl propionic acid-d4) as an internal reference was obtained from Acros Organics, New Jersey, USA.
Sample preparation
Extraction: Dried cannabis flowers were ground into a powder using a high-speed multifunction grinder (HC-1500Y). A mixture of 0.33 ml methanol and 0.17 ml water was combined with 0.5 ml of chloroform and added to 4 mg to 6 mg of the ground cannabis. The mixture was vortexed for five minutes and then centrifuged in a micro 2 Litres centrifuge at 14.8 × 103 rpm for 10 minutes at ambient temperature. The resulting solution was separated into three layers: The top (polar) layer containing the methanol/water solvent, the bottom (non-polar) layer containing the chloroform solvent, and the middle (solid) layer that dissolves exclusively in DMSO. The organic phase was transferred to a separate eppendorf tube and concentrated using a savant speedvac SPD1030 integrated vacuum concentrator at ambient temperature and a pressure of 6 torr for 4 hours-6 hours.
UPLC-MS/MS: The resulting extract was vortexed for 1 minute and filtered through a 0.22 µm filter unit. The solute was diluted to a ratio of 1:100, and 100 µl of the sample was transferred to LCMS vials and centrifuged for 5 minutes. The UPLC was performed using an aquity U-PLC BEH C18 analytical column with a linear gradient elution system consisting of eluant (I) at 50% and eluant (II) at 50% for 1 minute, II at 100% for 8 minutes, II at 50% for 3 minutes, and equilibrated for 2 minutes. The UPLC program ran for 13 minutes at a flow rate of 5 ml/min, and 0.5 µL of the sample was injected. The UPLC was quantified using an acquity-target lynx.
NMR: The non-polar phase was dissolved in deuterated chloroform (CDCl3) with 1% TSP as an internal reference at 0 ppm. The NMR spectra were recorded using a bruker ascend 400 MHz spectrometer at 25ºC. Various mixing times (0.03 seconds, 0.08 seconds, and 0.12 seconds) and 256 scans were used for the analysis. The NMR signals from protons in the sample were obtained using a standard non-phase-sensitive sequence (1D) and homo-nuclear shift correlation generated two-dimensional NMR experiments with 2k × 256 data points matrix.
Instrumental
Liquid Chromatographic (LC) conditions: Analytes were separated on an aquity U-PLC BEH C18 analytical column preceded by an acquity U-PLC BEH C18 vanGuard pre-column. The flow rate was 0.5 ml/min, and the autosampler and analytical column temperatures were maintained at 10ºC and 45ºC, respectively. The mobile phases consisted of 0.1% formic acid in water (I) and 0.1% formic acid in acetonitrile (II).
Mass spectrometry conditions: The quadrupole time-of-flight tandem mass spectrometer system (waters SYNAPT G2-Si Q-ToF) was used with Electrospray Ionization (ESI) in positive and negative modes. The mass spectrometry parameters included capillary voltage of 1.50 kV, collision gas flow of 0.15 ml/min, extractor voltage of 3 V, desolation temperature of 500ºC, source temperature of 150ºC, and desolation gas flow of 1000 l/h. The mass scan ranged from 50 m/z to 1200 m/z.
Biological activity of cannabis extracts on glioblastoma cell line culture
Each compound was dissolved in DMSO and tested on cells. The supernatant sample was obtained by spinning the compound with DMSO and the pellet at 3000 RPM for 5 minutes. The leftover DMSO was evaporated from the open microfuge tubes containing the pellets on a 37ºC heat block. The mass of compounds 1 and 2 was obtained using the maximum tolerable amount of DMSO (1%) and further diluted at a 1:10 ratio. Compound #2 or the non-polar layer was further tested with a 1:2 serial dilution of DMSO concentration starting at 1% DMSO.
The scientific paper presents a comprehensive investigation into the analysis of cannabis extracts and their impact on glioblastoma cell lines. The study utilized three instrumental techniques, namely Liquid Chromatography-Mass Spectrometry (LC-MS) and Nuclear Magnetic Resonance (NMR).
Liquid Chromatography-Mass Spectrometry (LC-MS) is a vital tool in modern analytical chemistry for identifying chemical compositions. It combines the separation capability of liquid chromatography with the sensitive and selective detection power of mass spectrometry. By analysing parent ions, daughter fragmentations, and retention times of compounds, LC-MS enables the confident identification of each compound. Parent ions provide information about intact molecular species, while daughter fragmentations offer insights into structural characteristics. The retention times aid in distinguishing similar compounds with distinct mass spectra, facilitating compound characterization.
In the LC analysis, an aquity U-PLC BEH C18 analytical column, preceded by an acquity U-PLC BEH C18 vanguard pre-column, was employed. The mobile phases consisted of 0.1% formic acid in water and 0.1% formic acid in acetonitrile. LC was coupled with a waters SYNAPT G2-Si Q-ToF tandem mass spectrometer system for mass spectrometry. The LC-MS conditions were optimized using tandem MS/MS ions for each standard solution of cannabis metabolites. Figure 1 displays the LC-MS chromatogram representing the methanol extract, while Table 1 summarizes the chromatogram LC-MS results, including retention time and m/z values for parent and daughter fragmentations. Additionally, UPLC-MS/MS analysis using methanol/water extraction yielded a chromatogram (Figure 2) identifying various compounds present in the extract, such as Cannabigerol (CBG), Cannabichromene (CBC), Cannabidiol (CBD), Delta-9-Tetrahydrocannabinolic acid ( Δ-9-THCA), CBGA (Cannabigerolic Acid), CBG (Cannabigerol), CBD (Cannabidiol), THCV (Tetrahydrocannabivarin), CBN (Cannabinol), Δ-8-THC (Delta-8-Tetrahydrocannabinol), Δ-9-THC (Delta-9-Tetrahydrocannabinol), CBC (Cannabichromene), CBDV (Cannabidivarin). The polar solvent system extract and ion masses confirmed the presence of these cannabinoids in the extract through high-resolution mass spectrum and retention times, respectively. The UPLC-MS/MS results align with the findings from NMR analyses, providing further verification of the identified compounds in the cannabis extract.
| Name | Retention time (min) | MSMS transitions | Chemical structure | Quantification of limit (LOQ) | LCMS | NMR |
|---|---|---|---|---|---|---|
| ÃŽÃâ9 THCA | 6.4 | 357.2101 ÃÆà313.2145 | 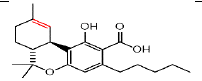 |
25 | Yes | Yes |
| CBDA | 4.3 | 357.2066 ÃÆÃÂ 245.1538 | 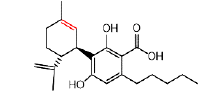 |
10 | Yes | Yes |
| CBGA | 4.5 | 359.2192 ÃÆÃÂ 341.2126 | 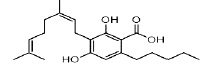 |
10 | Yes | Yes |
| CBG | 4.5 | 317.2470 ÃÆÃÂ 193.1223 | 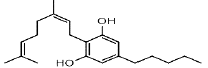 |
50 | Yes | Yes |
| CBD | 4.6 | 315.2336 ÃÆÃÂ 259.1668 | 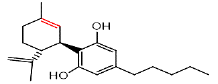 |
25 | Yes | Yes |
| THCV | 4.5 | 287.2031 ÃÆÃÂ 165.0929 | 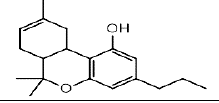 |
25 | Yes | Yes |
| CBN | 5.3 | 311.2011 ÃÆÃÂ 223.1130 | 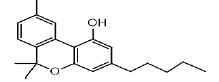 |
10 | Yes | Yes |
| ÃŽÃâ8 THC | 5.91 | 315.2336 ÃÆà193.1242 | 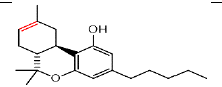 |
10 | Yes | Yes |
| ÃŽÃâ9 THC | 5.8 | 315.2324 ÃÆà259.1705 | 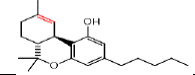 |
25 | Yes | Yes |
| CBC | 6.4 | 315.2336 ÃÆÃÂ 193.1242 | 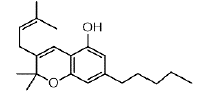 |
50 | Yes | Yes |
| CBDV | 3.4 | 287.19771 ÃÆÃÂ 65.0892 | 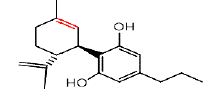 |
10 | Yes | Yes |
Table 1. Summarizes the LC-MS, GCMS, and NMR results for the identified compounds in the non-polar extract of Cannabis sativa.
The quantification of individual cannabinoids in gram quantities of dried cannabis flower yielded impressive results. Regarding the calculation of the concentrations, it is important to clarify that the concentrations were determined using the calibration curves generated in the previous method assay. The calibration curves were created based on the best-fit linear regression method.
Each calibration curve was prepared using six concentrations of each cannabinoid, with each concentration repeated five times. These calibration curves were then saved and utilized for the quantification process. To calculate the cannabinoid concentrations in the samples, the software (Target Lynx) integrated within mass lynx was employed. The software utilizes the calibration curves to determine the concentration of cannabinoids in the samples based on their respective peak areas. This method allows for accurate quantification of cannabinoids by incorporating the calibration curves developed using the UPLC-MS/MS method.
Among the cannabinoids analyzed, Cannabichromene (CBC) emerged as the most prominent component, constituting an impressive 38% of the total. Notably, cannabigerolic acid.
Cannabigerolic acid (CBGA), Cannabigerol (CBG), and Cannabidiol (CBD) were also present in substantial amounts, comprising 14%, 8%, and 6%, respectively (Figure 2). To gain deeper insights into the complex organic extracts chemical composition and structural elucidation, we employed proton nuclear magnetic resonance spectroscopy (1H-NMR). This powerful technique allowed us to identify and quantify various organic compounds based on their unique proton chemical shifts, shedding light on the extracts functional groups. Additionally, the selective TOCSY experiment enabled the determination of proton connectivity, facilitating the assignment of intricate proton spin systems and enhancing molecular structure elucidation (data not shown). Together, these cutting-edge methods provide a robust toolset for characterizing and comprehending the intricate mixture of organic compounds within the extract, opening new avenues for natural product discovery and environmental analysis.
In our 1H-NMR spectrum, acquired using a standard pulse sequence, we further performed 2D experiments, such as TOCSY, to gather additional spectral information. The NMR analysis yielded valuable structural insights and confirmed the identification of the compounds listed in Table 1. For instance, delta-9-tetrahydrocannabinolic acid (Δ 9THCA) exhibited chemical shifts at 6.2 ppm for aromatic protons and 4.5 ppm for aliphatic protons, while the ion mass parent and daughters were verified through high-resolution mass spectrometry (HRMS-LCMS) as 357.2101 and 313.2145, respectively. Similarly, CBGA, CBG, CBD, THCV, CBN, Delta-8-Tetrahydrocannabinol (Δ-8-THC), Delta-9-Tetrahydrocannabinol (Δ-9-THC), CBC, and Cannabidivarin (CBDV) were all successfully identified and confirmed through this comprehensive NMR analysis (Figure 3).
This extensive characterization of cannabinoid compounds lays a solid foundation for further studies and applications in the realm of chemistry and beyond.
In this chemistry journal, we conducted an evaluation of the biological activity of cannabis extracts on glioblastoma cell lines. To assess their impact, the extracts were dissolved in Dimethyl Sulfoxide (DMSO) and subjected to cell testing. Remarkably, the non-polar extract demonstrated a potent cytotoxic effect on the U250 glioblastoma cell line, showcasing its potential as a promising therapeutic agent (data not available). However, the results took an intriguing turn when the polar, nonpolar, and middle layers of the cannabis extract were tested on the U570 glioblastoma cell line (Figure 4). Surprisingly, none of these extract layers exhibited any toxic effects on the U570 cell line. This finding raised questions about the potential mechanisms at play, prompting us to delve further into the analytical chemistry analysis.
Figure 4: A) Pharmacological activity of Cannabis sativa extracts in polar and non-polar solvents and the middle-layer extracts. Those extracted were tested and incubated with two different glioblastoma cell line cultures U570. We did not see any toxic effect on cancer cell line culture using polar, non-polar and middle-layer extracts. B) Shows that individual cannabinoid has potent cytotoxic effect on U570 cell line culture. 


Our analytical chemistry analysis revealed that the polar extract predominantly contained Cannabichromene (CBC) and Cannabigerolic Acid (CBGA) as major constituents, comprising 38% and 14%, respectively. To explore the implications of these individual components, we tested them in pure form on the cell line culture. Interestingly, when tested individually, CBC and CBGA showed no toxic effects on the cancer cell line.
This observation has significant implications for our understanding of the cannabis extracts biological activity on glioblastoma cell lines. The lack of toxic activity in the polar extract, despite its composition of CBC and CBGA, suggests potential synergistic effects or interactions with other compounds within the extract. Further investigations into the intricate interplay of cannabis extract constituents may shed light on its therapeutic potential and guide future research in the quest for novel glioblastoma treatments.
The presented results unravel a captivating glimpse into the chemical composition and pharmacological potential of cannabis extracts. By harnessing the power of advanced instrumental analysis techniques such as Nuclear Magnetic Resonance (NMR) and Ultra-Performance Liquid Chromatography-Mass Spectrometry (UPLC-MS/MS), we embarked on a journey to identify and unravel the diverse compounds within these extracts. The combination of LC-MS and NMR allowed us to not only identify and quantify specific compounds but also to gain deeper confirmation of their presence.
The biological activity testing on glioblastoma cell lines yielded intriguing results. While the extracts did not exhibit potent cytotoxic effects on the U750 cell line, we uncovered the potent anticancer activity of certain cannabinoids such as CBD, CBDA, CBG, Δ-8-THC and Δ-9-THC. These promising findings highlight the cannabis extracts remarkable potential as an effective anticancer agent, offering hope in the quest for innovative treatments against this devastating disease. However, it is noteworthy that the polar extracts, containing relatively lower levels of these potent cannabinoids, did not display any toxic effects on the tested cell lines. This revelation piques our curiosity, prompting us to explore the intricate interplay of the cannabis extracts constituents and their synergistic effects..
The knowledge garnered from this study significantly advances our understanding of the chemical composition and biological activity of cannabis extracts, especially concerning glioblastoma cell lines. This breakthrough paves the way for further in-depth research and investigations, unveiling the precise mechanisms of action and unlocking the full therapeutic potential of these extracts. The potential therapeutic and pharmacological implications of the identified compounds, such as CBD, CBDA, CBG, Δ-8-THC and Δ-9-THC, are truly promising. As we continue to delve into their multifaceted properties, we envision a future where these compounds may revolutionize medical and pharmaceutical contexts, ushering in ground breaking treatments for various ailments. In conclusion, the instrumental analysis techniques of NMR, HRMS, and UPLC-MS/MS have provided us with a comprehensive understanding of the chemical composition of cannabis extracts. The revelation of potent anticancer properties within specific cannabinoids fuels our determination to explore these extracts full potential and design novel therapeutic solutions to combat glioblastoma and other medical challenges. The journey has just begun, and with unwavering dedication, we embark on a path to harnessing the true power of cannabis extracts in the realm of medicine and beyond.
Ashraf Duzan: Conceptualization, methodology, software, data curation, writing original draft preparation, Waldemar Debinski: Has done the biological activity, Mufeed Basti and Ashraf Duzan: Have done the NMR exteriments, Ashraf Duzan: Writing- Reviewing and Editing.
The authors declare no competing financial interest.
No fund for this review. This research completed at the research Lab at Wingate Pharmacy School, Wingate University and North Carolina State University of Agriculture and Technology.