ISSN: 2319-9873
ISSN: 2319-9873
Abdul Karim Shah1*, Zeenat Muhammad Ali2, Abdul Jabbar Laghari3, and Syed Farman Ali Shah2
1Chemical Engineering Department, Dawood University of Engineering and Technology, Karachi, Pakistan.
2Chemical Engineering Department, Mehran University of Engineering and Technology, Jamshoro, Pakistan.
3Dr. M. A. Kazi Institute of Chemistry. University of Sindh, Jamshoro. Pakistan
Received: 30/12/2012; Revised: 04/02/2013; Accepted: 05/02/2013
Visit for more related articles at Research & Reviews: Journal of Engineering and Technology
Coal and sugar manufacturing power generation plants are engender million tons of fly ash as waste per annum. It creates serious disposal and environmental problems. There is no alternative usage for its utilization in industries. In this regard, efforts were taken to utilize fly ash waste in the treatment of highly toxic and polluted dyes effluents. In this advanced research, characterization of fly ash properties, preparation of adsorbent, utilization for the optimum reduction of dyes effluent pollutants, determination of adsorptive capacity and study of isotherm adsorption models were accomplished. Treatment efficiency was optimized using these ashes as adsorbent at optimum dose. Sugarcane bagasse fly ash (SBFA) could reduce the higher concentration of COD (51%), color (70%), turbidity (71%) and TSS (96%) from dyes effluent. All used fly ashes could reduce higher concentration of effluent pollutants at 4 g dosing. SBFA has high porosity, which resulted in high adsorption of effluent pollutants as compared to other fly ashes. The adsorptive capacity of all used fly ash was declined on increasing adsorbent dosing. Langmuir and freundlich isotherm models were evaluated for the determination of chemical adsorption behavior of fly ashes
Wastewater Treatment, Adsorption, Wastewater Pollutants, Fly Ash adsorbents
Dyes manufacturing and textiles industries are one of the problematic groups due to disposing of highly toxic industrial effluents due to presence of toxic dyes and chemicals, which creates the harmful effects on marine and environment. It was reported that these industries are disposing approximately 100 tons of toxic dyes into effluents streams annually [1]. Recently, the annum worldwide production and consumption of over 10,000 different dyes have been exceeded 7*105 tons [2]. The major consumption of water in industries is due to processing and cleaning operations of chemical process equipments. These industries dispose the highly complex and toxic wastewater into rivers and sea without treatment [3]. In Jamshoro region, dyes manufacturing plants are producing 1200m3 per day highly complex and toxic effluents and disposing into rivers without any effective treatment. Dyes effluents contains high range of color, biological oxygen demand (BOD), chemical oxygen demand (COD), turbidity, pH, temperature, alkaline substances, acids, heavy metals and toxic substances [4]. The characteristics of dyes effluents vary according to each dye product manufacturing [5]. Discharge of such dyes effluents in rivers causes environmental pollution and produces allergic diseases [6,7]. It was declared from dyes manufacturing plant effluents analysis that industrial effluents contain high concentration of organic pollutants such as dyes, phenolic compounds, BOD, COD, color, TDS, turbidity, suspended solids and heavy metals. Common treatment technique (flocculation) was unsuitable for the treatment of such effluents due to their complexity.
Adsorption is an effective method for the separation of effluent pollutants and effluents treatment. It is used for the diminution of effluent pollutants, organic and toxic compounds effectively [8]. Adsorption method for color elimination is based on the affinity of various dyes for adsorbents. Adsorption capacity of adsorbent principally depends on the characteristics of material as specific surface area, pore size, and its distribution [9]. The powdered activated carbon (PAC) is the appropriate adsorbent for effective treatment of dyes effluent, but desorption and regeneration are difficult, uneconomical and expensive [10].
Coal fly ash, a waste generated from coal power plants during burning of powdered lignite coal into fluidized bed combustion technology system for the power generation. This powdered form waste creates serious environmental, disposal and health problems [11]. This waste worldwide production is about 600 millions tones per annum and Pakistan generates 55680 m3/hr [12].
Coal fly ash is grey in color, abrasive, refractory and acidic in nature. It has specific surface area in the range of 2500 and 7000 cm2/g, porosity (0.38%), pore volume (0.023 cm3/g), particle size of 120-960μm to less than 5 μm, specific gravity of 2.3-2.5 and bulk density of 600-900 kg/m3 [13].
The worldwide researchers have taken effective efforts for the utilization of fly ash into effective industrial applications for the resolution of wastewater treatment problems [14].
The annually production of sugarcane fly ash at world level is 47,800 millions tones and Pakistan baggase ash generation share is about 0.5 millions tones [15]. This ash has porosity (0.36%), pore volume (0.1067 cm3/g), surface area (168.39 m2/g) [16]. It has good porous structure and adsorption capacity. Many researchers have developed low cost fly ash adsorbent for the removal of phenolic compound, heavy toxic metals [17], dyes [18], organic pollutants and organic acids [19] from industrial flue gases and effluents.
Purpose of this advanced research was to optimize and compare the treatment efficiency of fly ash adsorbents for reduction of dyes pollutants from industrial dyes effluents via adsorption technique using coal power plant’s waste.
Low cost adsorbents were prepared from collected fly ash samples and were utilized for the treatment of industrial dyes effluent for an effective reduction of organic pollutants in the course of adsorption technique. Various samples of coal ash were together from FBC boiler of LPGCL, Jamshoro. Samples of SBFA were collected from boiler of power generation plant of Habib Sugar Mills (Pvt.) Limited, Nawabshah. The material composition of collected fly ash samples were analyzed at Fuel Research Laboratory PCSIR, Karachi.
These ash samples were converted into fine powder product (mesh 200) by sieving operation using RO-Tap Type Sieve shaker (A-871205, Heiko Seisakusho Tokyo, Japan) at 290 rpm speed.
The fine powdered ash was treated by physico-chemically method. In 1st instant, the ash samples were washed with warm distilled water (60 0C) for 5 minutes. The diluted solution of sulfuric acid (10% concentrated) was prepared for the washing of ash samples. This chemical washing was performed for 5 minutes using vacuum filtration system. Again ash samples were washed with warm distilled water and samples were kept into Oven (Lo-201C, The Grieve Corporation, USA) at 110 0C for drying and adsorbent activation purpose [20].
SEM analysis of these raw and prepared materials was performed by Scanning Electron Microscope (SEM) (JSM-6380, JEOL Ltd, Tokyo, Japan) by applying 05KV electron acceleration voltage at Advanced Research Laboratory, MUET.
The wastewater samples were collected from the main disposal point of dyes manufacturing industry and collected samples were kept in laboratory for analysis. The specific wastewater pollutants parameters were measured according to the standard laboratory protocols. COD of effluent samples was measured through dichromate method using COD vial, COD heater (TR-320, Merck, USA) and spectrophotometer (DR-2000, Hach, U.S) using by adjusting stored COD program 435 and wavelength 620 nm. Color, turbidity and TSS of dyes effluents were measured through absorptometric method using Spectrophotometer at wavelengths by 455 nm, 450 nm and 810 nm respectively.
Effluent samples volume was measured through volumetric measuring cylinder and was poured into a number of Erlenmeyer flasks (100ml). Entity quantities of all prepared fly ash adsorbents were measured through analytical balance and were poured directly into each effluent samples in Erlenmeyer flasks. Hot plate magnetic stirrer was used for proper mixing and adsorption rate of fly ash adsorbents in effluent samples. Process parameters such as agitation time 5 minutes, speed 150 rpm, settling time one hour and temperature 30ºC were maintained. After treatment, samples of treated effluent were filtered via vacuum filtration system using whatman filter paper (125 μm). Finally quality parameters of treated effluent samples were analyzed according to standard laboratory protocols. The comparative study was conducted for all fly ash samples’ adsorption capacity in effluent treatment [20].
The adsorption capacity of all prepared adsorbents was determined regarding to the pollutants adsorption from dyes effluents. In this case, contact time, adsorption equilibrium and constant agitation speed were considered in the adsorption process. The adsorption (%) of effluent pollutants on fly ash adsorbents was calculated as follows:
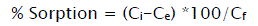 (1)
(1)
Where Ci (mg/l) is the initial concentration of effluent pollutants before adsorption and Ce (mg/l) is the concentration of pollutants at equilibrium state [21].
In same way, the adsorption capacity (mg/g) was calculated as follows:
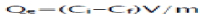 (2)
(2)
Where Qe is the adsorption capacity (mg/g), Ci and Ce is the initial and equilibrium concentration (mg/l) of pollutants in dyes effluent respectively, V is the volume (mL) of effluent and m the mass (g) of fly ash adsorbent [22].
Material composition and SEM analysis of fly ash adsorbents
The adsorption is the process of absorbing pollutants into the pores of adsorbent. The rate of adsorption depend upon surface area, pore size, surface area and composition [23]. Material composition showed that these fly ashes have suitable chemical composition. Oxides of alumina, silica and iron in fly ash behaved also as good coagulants for the reduction of effluent pollutant’s concentration. Due to high surface area, porosity and adsorption capacity, fly ashes worked as activated carbon adsorbents. The percentage of oxides of iron and aluminum was high in material composition of fly ashes. Fly ashes’ chemical behavior proved effective for the treatment of industrial effluent (Table 1). Sugarcane bagasse fly ash has high porosity and surface area than coal ashes. When coal fly ashes have high surface area and less porosity. High porosity and surface area results in high adsorption capacity for pollutants removal [24]. Physico-chemical treatment could enhance adsorption capacity of prepared adsorbent (Fig.1). Adsorption capacity was mainly due to their structural characteristics and their porous texture which gives them a large surface area, and their chemical nature which can be easily modified by chemical treatment in order to increase their adsorptive properties [25]. Effective effluent treatment via adsorption depends upon porosity, chemical structure, particle size, surface area and adsorption capacity of fly ashes.
Adsorptive capacity and adsorption (%) for the adsorption of pollutants by use of fly ash adsorbents
Fly ash adsorptive capacity allowed reduction of pollutants from dyes wastewater. The prepared adsorbents have good porosity and adsorption capacity and could reduce the concentration of suspended solids by behaving as a settling aid. Industrial dyes effluent sample (100ml) was treated via adsorption process using prepared adsorbent from fly ashes at dose variations for removal of effluent pollutants. The experimental study declared that SBFA adsorbent could adsorb maximum percentage of effluent pollutants such as COD, color, turbidity and TSS. From coal fly ash type’s adsorption study, it was concluded that CFA has high potential for adsorption of effluent pollutants, but less than SBFA as compared. Maximum adsorption rate was found in TSS removal in all used fly ashes (Table 2). In same way, adsorptive capacities (mg/g) of all used fly ashes adsorbents were found. Industrial effluent was treated via adsorption method using ash adsorbents at different dosing for dyes effluent pollutants reduction.
Comparative study for COD removal from effluent via adsorption technique
The initial COD concentration of collected dyes effluent sample was 1164 mg/lit. Industrial dyes effluent samples (100 ml) were treated through prepared ash adsorbents for effluent’s COD removal at different doses. SBFA dose (4g) and CFA dose (8g) could reduce COD by 51% and 49% respectively. Equilibrium state condition was observed in increasing adsorbent dose and uniform removal rate was observed. BCA (2g) and CCA (4g) could reduce higher COD rate by 39% and 44% respectively (Fig.2b). From the experimental study, SBFA showed good performance in COD reduction SBFA gave the most effective results than coal fly ashes because SBFA has high porosity and could capture organic compounds effectively. Srivastava et al. (2005) reported removal rate of COD (50%) of pulp mill wastewater using sugarcane bagasse fly ash adsorbent at pH 4 and BFA dosage of 2 g/l [26]. Adsorption capacity depends upon the interface of effluent pollutants with the prepared material from fly ashes. The presence of inorganic salts could enhance the adsorption of organic species to carbon. These salt ions of activated carbon which carried out charges opposite to that of the adsorbed organic ions were attracted to the spaces between adjacently adsorbed organic ions and could reduce the strength of repulsion of the adjacently adsorbed organic ions [27]. Sun et al. (2008), reduced COD (44%) from paper making wastewater using bottom coal ash at 8g/100ml dosage [28]. Coal fly ashes showed less effect on pH reduction. pH range was under NEQS. pH rate declined vs. SBFA adsorbent dosage and result in effluent in acidic nature. Lime as pH maintaining agent should be used for maintenance of pH of effluent.
Comparative study for color removal from effluent via adsorption technique
The prepared ash adsorbents were utilized at dose variations for the dyes effluent treatment in order to reduce pollutant’s concentration. The initial dyes color concentration of collected dyes effluent sample was 6560 ptco. SBFA (4g) and CCA (8g) could reduce effluent color by 70% and 535 respectively. At 8g dose, CFA and BCA could reduce 53% and 51% color of dyes effluent. At optimum dosing of SBFA could reduce higher rate of color of effluent. Due to porosity, chemical composition and specific surface area of fly ashes, high rate of color could be reduced along with adsorbent dose rate. Rao et al (2006) reduced color (96%) from textile effluent at higher dose (12g) of fly ash [29]. High porosity could capture the coloring pollutants of effluent effectively in fly ashes pores. SBFA SEM analysis declared that it has high porosity and can capture organic pollutants due to its high porosity and inorganic salts presence. Dyes coloring compounds were break up and captured by pores of fly ashes as well as elemental chemical behavior of fly ashes. Adsorptive capacity of fly ash for COD adsorption declined on increasing adsorbent dosing. Steady state condition was observed in higher dosing rate.
Comparative study for turbidity removal from effluent via adsorption technique
The initial turbidity concentration of collected dyes effluent sample was 1280 FTU. The optimum dose (4g) of SBFA and CCA could reduce turbidity by 71% and 53% respectively. The optimum dosing (8g) of CFA and BCA could reduce color by 55% and 53% respectively (Fig.4(b)). Turbid material of effluent was captured in the pores of fly ash adsorbent. SBFA has high porosity and will result good performance in effluent pollutants reduction. Coal fly ash has high surface area and also reduced the turbid material concentration from effluent. When adsorption equilibrium occurs, maximum contact time did not reduce turbidity rate of effluent. When adsorption equilibrium occurred, it stopped the adsorption of turbid material. Adsorptive capacity of fly ash for color adsorption declined on increasing adsorbent dosing. At lower dose (2g/100ml), higher adsorptive capacity of fly ash for color adsorption was recorded. Steady state condition was observed in higher dosing rate.
Comparative study for TSS removal from effluent via adsorption technique
The initial suspended solids concentration of collected dyes effluent sample was 349 mg/lit. The optimum dosing (8g) of SBFA, CFA, BCA and CCA could reduce TSS by 96%, 92%, 87% and 91% respectively. High porosity of material enables it to adsorb higher rate of suspended solids. According to adsorption capacity of adsorbent, equilibrium state will achieved within specific contact time. SBFA adsorbent reduced maximum concentration of suspended solids from effluent due to its high porosity. Suspended solids were interacted with fly ash pores and surface area and were captured in fly ashes pores, until equilibrium condition was achieved. The adsorptive capacity of fly ashes improved by physico-chemical treatment. In this case, higher turbidity removal rate was observed at lowest dosage (2g). On increasing dosing ratio, steady state condition was observed in higher doses (6-8g).
The adsorption behavior of adsorbents was determined by statistical analysis using isotherm models of Langmuir and Fruendlich. These models determined liquid/solid phase sorption behavior, sorption capacity, structure of sorbed layer and interaction between sorbet and sorbent. Langmuir isotherm assumes monolayer coverage of the adsorbate on a homogeneous surface of adsorbent, where as Fruendlich isotherm assume the heterogeneity.
The experimental data have been analyzed by using the linear forms of Langmuir and Fruendlich isotherm models in the following way:
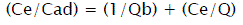 (3)
(3)
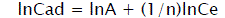 (4)
(4)
Where Cads is the amount of sorbate (mg/g) at the sorbent surface and Ce is the amount of sorbate in the liquid phase at equilibrium (mg/l). 1/n is sorption intensity, b is Langmuir constant (l/mg), and Q is monolayer sorption capacity (mg/g).
RL value indicate favorability (0<RL<1) of isotherm. RL=0; irreversible isotherm, 0<RL<1; favorable isotherm, RL=1: linear isotherm, RL>1: unfavorable isotherm.
The value of sorption intensity (1<n<10) indicates that sorption is favorable. The magnitude of sorption isotherm constants, gives an indication of favorability and unavoidability of sorption process. The values of Q and Langmuir constant b are calculated from the slope and intercept of the plot of Ce/Cads vs. Ce [30]. Adsorption correlation co-efficient (R2) and other isotherm constants were calculated for all used fly ash for adsorption of dyes effluent pollutants such as COD, color, turbidity and TSS. In this study, two well known models of Langmuir and Freundlich isotherm would be evaluated.
From the models study, it was concluded that Freundlich isotherm model was found suitable for adsorption of dyes effluent pollutants by use of coal fly ash. Adsorption correlation coefficient (R2) was higher in Fruendlich isotherm model. In same way, Fruendlich model was also fit for BBCA adsorbent for pollutants reduction. When Langmuir model was fit for SBFA adsorbent in treatment study. In case of CCA adsorbent adsorption study, two parameters such as COD and TSS has higher R2 (0.904 and 0.965) values in Fruendlich model. Freundlich isotherm model justified that there was multilayer formation on sorbent surfaces of CFA and BBCA and Langmuir isotherm model justified that there was monolayer layer on the sorbent surface of SBFA.
The comparative study of fly ashes adsorption for dyes effluent pollutants was conducted. It was concluded that all used fly ashes have good adsorption capacity for COD, color, turbidity and TSS. CFA, BCA, CCA and SBFA adsorbents declared the effective reduction rates of color (53%, 51%, 53% and 70%), COD (49%, 37%, 44% and 51%), turbidity (55%, 53%, 54% and 71%) and total suspended solids (92%, 87%, 91% and 96%) respectively. It was concluded that SBFA has good porosity and adsorption capacity and was effective for dyes effluent treatment. The adsorptive capacity of all fly ash adsorbent declines vs. adsorbent dose. Adsorption rate improved in ever-increasing adsorbent dose. The pH of effluent declined in ever increasing adsorbent dosing. Freundlich isotherm model was fit for CFA and BBCA and showed multilayer adsorption behavior. Langmuir isotherm model was fit for SBFA adsorbent and declared monolayer adsorption behavior in treatment of dyes effluent.
It was recommended that coal power plant’s waste must be utilized as low cost adsorbent for the effective and economical treatment of industrial dyes effluent.
✓ COD: Chemical Oxygen demand
✓ TDS: Total Dissolved Solids
✓ TSS: Total Suspended Solids
✓ CFA: Coal Fly Ash
✓ SBFA: Sugarcane Bagasse Fly Ash
✓ BBCA: Bottom Based Coal Ash
✓ CCA: Cinder Coal Ash
The authors would like to thanks to Management of Clariant Pakistan Limited, Jamshoro for providing research facilities at Effluent treatment Plant Laboratory.