ISSN: 2347-7830
ISSN: 2347-7830
Emmanuel HL1, Peter K2* and Alex WK3
1Private Research Consultant, Moshi, Tanzania
2Peter Kiffney, Northwest Fisheries Science Centre Washington USA 10 Park Avenue, Building B, Mukilteo, Washington 98275, USA
3College of African Wildlife Management, Mweka, Moshi, Kilimanjaro, Tanzania
Received date: 01/5/2015; Accepted date: 23/6/2015; Published date: 29/6/2015
Visit for more related articles at Research & Reviews: Journal of Ecology and Environmental Sciences
We hypothesized that variation in physical and chemical properties and habitat destruction of the Themi River as a result of human activities would affect abundance and diversity of amphibian and benthic macroinvertebrates. Variation in habitat physical and chemical conditions, and amphibian and benthic macroinvertebrate diversity and abundance were assessed in the Themi River of Arusha municipality. These physical, chemical and biological conditions were assessed at forty sampling stations across four river sections (monitoring sites) that were located above and below municipal discharges. Water chemistry was evaluated by measuring the concentrations of SO4, Cu2+, Cl-, Cr6+, NO3 -, PO4 3- NH4+, turbidity, dissolved oxygen, water hardness (CaCO3) and pH. Water velocity, depth, width, temperature and damaged area were also measured. Shannon-wiener (H’) diversity, abundance and habitat destruction varied significantly among sampling stations. Diversity of amphibia and benthic macroinvertebrates decreased with increased habitat destruction, and changes in the chemical and physical properties of the river. These changes corresponded to changes in land use and discharges associated with human activities. Fresh water is crucial resource for humans and freshwater organisms in the Themi River. This study suggests that benthic macroinvertebrates and amphibians can be used to monitor human impacts on the Themi River and other rivers in Tanzania.
Amphibians, Benthic Invertebrates, Biodiversity, Biomonitoring, Chemical, Contaminants, Habitat Damage, Rivers.
Watershed Pollution
Water plays a fundamental role in our daily life: drinking, bathing, cooking, washing, farming, garden irrigation, transportation, industrial raw materials, recreation and sport, production of hydroelectric power, building construction, and agriculture and wildlife survival [1-3].
There are significant pressures on freshwater ecosystems caused by human activities. Water availability and quality are deteriorating due to climate change and land use activities (mining, logging, industrial, sewage discharges) [4]. These activities introduce hazardous chemicals that suppress populations and diversity of aquatic organisms [5,6].
In Tanzania the major types of water pollution related to human activities are urbanization, industrialization, mining activities, poor management of heavy metals, and sewage systems (Moss, 1980). Both organic and inorganic pollutants are discharged to most urban rivers in developing countries especially in highly populated regions. Benzene (C6H6), ammonium nitrogen (NH3–N), phosphate (PO4), sulphate (SO4), chloride (Cl), magnesium (Mg), zinc (Zn), and lead (Pb) are among the common chemicals found in fresh water ecosystems that receive anthropogenic discharges [7-9].
Physical characteristics of river systems and human alteration of these characteristics are among the other factors that influence biodiversity, population size and stream productivity of fresh water organisms i.e. fish, micro/macroinvertebrates and frogs [10-12]. These factors include water velocity, turbidity, river depth and width as aquatic species generally select physical conditions that promote growth, survival and reproduction [13-17].
Freshwater organisms can, therefore, be sensitive to habitat destruction, and physical and chemical properties of their environment, hence they are commonly employed as biological indicators [18-19].
Clements and Kiffney, reported that benthic invertebrates communities were altered at sites where zinc exceeded the EPA US water quality standards. Furthermore, Ferrari reported that tadpole’s (Bufo arenarum) mortality rate was 53% at nominal concentrations of 4 ppm cadmium chloride. Mwingira, in a study of urban wetland pollution in Dar es Salaam region (Tanzania), reported that, pH and biological oxygen demand of the Kiziga River was higher than the criteria required by national standards, which contributed to the loss of aquatic organisms compared to nearby reference streams.
Pollution in Themi River
The Themi River runs through the town of Arusha with population of 1,288,088 and growth rate of 4.0% annually. Therefore a growing population and industries threaten habitat suitability and productivity of this urban river [20]. For example, nutrients in municipal discharge can cause the formation of algal blooms, which leads to deoxygenating of stream habitat that can reduce the fitness of stream organisms [21]. It is difficult to isolate which factor or factors influence the diversity and abundance of organisms in natural ecosystems: Kiffney and Clements acknowledge that, separating natural community structure from variation caused by anthropogenic disturbance is one of the greatest challenges in stream biomonitoring studies. However, field studies provide an opportunity to investigate whether the distribution and abundance of organisms changes in the face of human modification of the landscape.
Residences and industrial sewage are discharged untreated into the Themi River. Other industries also discharge untreated effluent directly to Themi streams through concrete pipes and tributaries. Furthermore the stream is bordered by car washes, small farms and vegetable gardens, which potentially discharge chemicals into or damage river habitat. This situation motivated me to develop a study in this river ecosystem. My objectives were to identify the chemical and physical properties that may contribute to variation in abundance and diversity of amphibians and benthic macroinvertebrates of the Themi River; Specifically, we wanted to examine the evidence for human activities affecting chemical and physical properties of this system, which, in turn, alter species diversity and abundance of river organisms.
Study area
Themi River is located in the Arusha municipality, north east of Tanzania, at the latitudes of 2º and 6º south and longitudes 34.50º and 38º east. Annual temperatures range from 13º to 30ºC; during the wet season (February-June/October-December) the area receives from 1609 to 1825 mm and 425 to 745 mm of rainfall in the dry season (July-September) [22]. The study took place in between mid-May and late August 2011, this comprise of partly rain and dry seasons. Arusha experiences an eastern prevailing wind from the Indian Ocean, about 400 km east. A number of factories are located within the watershed including a brewery, tire production, fiberboard plant, and large pharmaceutical companies. The source of the Themi starts along the slopes of Mt. Meru at Olgilai village about 1700 m above sea level, and flows through Arusha town at an altitude of 1254 m [23]. The river joins the Kikuletwa River in Simanjiro district at Manyara region. The Themi is approximately 50-60 kilometres long.
Study design
To test the hypothesis that human activities affect the chemical, physical and biological conditions of the Themi River, we selected four monitoring sites along a 60 km section of the river (Figure 1). These sites have different characteristics in terms of how they are affected by human activities, and my hypothesis is addressed through assessment of the biological, physical and chemical variation at these sites. The following sites were identified: A and B, which were not affected by agricultural or sewage discharge; site C which was affected by industrial and sewage discharges, car washes and farms lands; and D, which was affected by agricultural and polluted water flowing from C. Site A started 2 km from Mt. Meru forest reserve, station B was 20 km from site A, site C was 25 km downstream of site B, and site D was 15 km from site C (Figure 1). Four transects were located, one at each monitoring site, and at each transect there were 10 sampling stations where we collected invertebrates and amphibians, and measured chemical and physical properties of the river. Therefore, there were a total of 40 sampling stations along the study area. The distance from one sampling station to another was determined randomly by using a generated random numbers in R.
Chemical properties
A total of 40 water samples (1500 ml each) were collected from the 40 sampling stations. These samples were brought to laboratory for analysis. The photochemical analysis of chloride, phosphate, nitrogen, ammonia, chromium, copper, sulfate and nitrate were conducted by DR/2010 HACH spectrophotometer [24]. The 1,5 diphenylcarbohydrazide method was used to analyze chromium hexavalent (Cr6+), Sulfa Ver 4 method for sulfate (SO4), salicylate method for ammonia (NH4+), cadmium reduction method for nitrate (NO3) and bicinchoninate method for copper (Cu2+); the detection limit for the spectrophotometer was between 0 and 0.001 mg/L (HACH, 1997). Water hardness (CaCO3) was assessed using titimetric method, pH was analyzed using Orion star A111 meter and dissolved oxygen determined by Extech 407510 dissolved oxygen meter.
Physical properties
Invertebrates generally display a clumped distribution, which is assumed to be related to the small-scale variability in a variety of factors including substrate composition, food abundance, flow conditions, and water depth [10,25]. Therefore, water temperature and velocity, stream depth and width, and turbidity were recorded at the forty sampling stations accordingly. These factors have been observed to influence and alter diversity and abundance of amphibians and benthic macroinvertebrates [7,10,26,27]. Stream temperature was measured manually using a glass thermometer [28]. Turbidity (NTU) was measured by AQUA fast AQ 3010 turbidity meter. Stream velocities were measured by digital hand-held current meter No: 445 500. Stream depth and width at each sample station were measured using a tape measure. Three measurements of each physical property were taken at each of the 40 stations.
Diversity and abundance
Benthic macroinvertebrates were collected using artificial substrate traps (cylindrical shape wire mesh; 75 cm circumference and 55 cm length); traps were filled with dry grasses and leaves [29]. Traps were placed at each transects and stations where water samples were collected; thus, there were total of 40 invertebrate trapping stations. Invertebrates traps were checked every 24 hours over a 96 hour period [30] After 96 hours, taps were collected and stored in 70% ethanol [7].
More than ten species of amphibia in the order Anura have been identified in Arusha National Park, which is in close proximity to the Themi river. These species include Bufo gutturalis (Guttural Toad), Xenopus muelleri (Mueller’s Clawed Frog), Ptychadena mascareniensis (Mascarene Grass Frog), Rana angolensis (Common River Frog), Strongylopus fasciatus merumontanus (Striped Long-toed Frog). Adult amphibians were were captured by drift fencing and pitfalls traps made of plastic buckets (diameter 30 cm, 40 cm height) (ibid). Two drift fences each with 10 pitfalls traps were located on each side of the river bank in the same transects where artificial substrates were placed; therefore there was a total of 80 drift fences and pitfalls locations. Drift fences were made of plastic sheeting (550 cm length and 100 cm height) that was held up by wooden poles (1.5 metre height). Pitfall traps were buried in the ground, with the opening flush with the ground surface. Traps were checked every day in the morning for 2 days [31-33]. To avoid vandalism, we recruited local community members to conduct help check trapping stations.
Habitat damage
A systematic sampling survey was applied to assess habitat damage caused by human activities at each of my monitoring sites (Figure 1). The upper site (A) had length of 20 km; the same length was assessed at the rest of the monitoring sites. Monitoring sites B and C had more than 20 km in length; therefore, we assessed the first 20 km of habitat starting at the boundary for each. Site D was 15 km long; therefore the entire length was surveyed. At each monitoring site we surveyed for human activity including trails, water diversions, vegetable gardens and sand mining. After identifying a disturbed section, we measured the area (length × width) of disturbed habitat including along the banks and in the stream.
Data analyses
Diversity and Relative Abundances: We used the Shannon-Wiener index of diversity to determine an adult amphibian and invertebrate diversity. Diversity and abundance were calculated for each sampling point (total of 40 points) whereby relative abundances were calculated based on pooling across individual samples at each monitoring sites (A, B, C and D). The relative abundances were obtained on Shannon wiener index calculations and recorded to the proportion of 0 to 1.
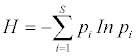
The Shannon Wiener Index formula
H’: - Shannon's diversity index
S: - Total number of species in the community (richness)
Pi: - Proportion of S made up of the i species
Lnpi: - Equitability (evenness)
Statistical data analysis: We used one-way analysis of variance (ANOVA, α= 0.05) to test the hypothesis that abundance and diversity of amphibians and benthic macroinvertebrates differed among the four monitoring sites [7,34,35]. There were many predictor variables (i.e., chemical properties) that were potentially correlated; therefore we ran principal component analysis in R to reduce the confounding effect of multiple correlated variables [36]. All chemical properties mentioned above were included in the principal component analysis; physical properties were excluded from the principal component analysis as there was little evidence of correlation among both physical and chemical variables. We used a generalized lineal model (GLM) and backward selection of physical properties together with principal components of chemical variables to determine whether they predicted variation in amphibian and invertebrate abundance and diversity. Before analysis, data were evaluated with respect to the assumptions of parametric statistics (i.e., independency of variables, normal distribution and equal variance). Some of the variables did not meet the assumptions; therefore, these variables were log-transformed (log and log+1). We used R (version 2.13.1) to run ANOVA and the multivariate model.
Among-Site Variation in Water Chemistry and Physical Conditions at the Themi River
The variation in mean chemical concentration at the four monitoring sites reveals the degree and approximate location of pollution sources along my study area. Values for most measured chemicals were relatively low at sites A and B relative to C and D. For example, heavy metals, such as chromium and copper, were detected at site C and D but not at sites A and B (Table 1). There was also a trend for values to be higher for some variables at B relative to A: Chloride (Cl-) was 6× higher at B relative to A.
In general, physical characteristics were similar to the spatial trend shown with chemical characteristics though not for all variables. Temperature, habitat damage, river width, and depth increased while water velocity decreased downstream. The area of damaged habitat and water temperature increased abruptly at site C relative to upstream sites (Table 2).
Amphibians and Benthic Macroinvertebrates
Ten species of amphibians (frogs) including 938 individuals were caught, identified and released back to the field. In addition, 14 benthic macroinvertebrates taxa and 4492 individuals were collected, identified and counted (Table 3).
Some amphibians were abundant at all sites (e.g., Ptychadena mascareniensis and Rana angolensis), while others were found only at site B (i.e., Hemisus marmoratum and Hyperolius viridiflavus ommatostictus). Other species were located only at site A and B (i.e., Strongylopus fasciatus and Hyperolius nasutus) (Table 3 and Figure 2).
A diversity of benthic macroinvertebrates was observed. Mayflies, caddis-flies, alderflies and whirligig beetles i.e. (Dinwutes spp. and (Aulonogyrus spp) appeared to be sensitive to human disturbance as they were basically found only at sites A and B. The earthworm was the only taxa collected at all monitoring sites. Midge flies, liverfluke and fresh water snails were common at sites C and D (Table 4 and Figure 3).
The ANOVA indicated no significant among site variation in amphibian abundances; mean abundances and standard errors indicated high overlap among sites (Figure 4).
However, the ANOVA model indicated significant among-site variation in benthic macroinvertebrates abundance χ²(3,36) 436.88, N=40, p=0.002). Invertebrate mean abundance was highest at C (mean abundance=132 individuals) followed by A, B and (108, 100 individuals), and lowest at D (52 individuals) (Figure 5).
Figure 5: Mean benthic macroinvertebrates abundances (± 95% CIs) collected at four sites at Themi River, Tanzania. Site A is upstream of the disturbed area, B was little disturbed, C was located in the middle of a highly disturbed and polluted section, while site D was the most downstream site and was also considered disturbed and polluted.
The ANOVA model also indicated significant among-site variation in amphibian (F (3,36)=1.91, p<0.001) and invertebrate (F(3,36)=3, p<0.001 ) diversity . Amhibian diversity was lowest at C and D compared to A and B; amphibians diversity at Site B was more than two times higher than sites C and D (Figure 6). Invertebate diversity showed a pattern similar to amphibian diversity whereby sites C and D had lowest diversity compare to sites A and B; site B was almost twice diverse as site C and D (Figure 7).
Relationships Between Physical-Chemical Properties of Amphibian and Macroinvertebrate Abundance and Diversity
The first principal component (PC1) explained 86.6% of the variance in chemical properties measured at my study sites; therefore, this variable was used as a predictor in the generalized linear model (Table 5).
Sulphate, chloride, chromium, nitrate, copper, phosphate, ammonium, turbidity (NTU), and hardness (CaCO3) were positively correlated with PC1, while pH and dissolved oxygen were negatively correlated (Table 6).
Water temperature was negatively associated with amphibian diversity, while river width was negatively associated with amphibian and macroinvertebrates abundance (Table 7).
PC1 and water temperature were significant predictors of benthic macroinvertebrate diversity, with both covariates negatively associated with invertebrate diversity (Table 7). The important chemicals variables contributing to PC1 were copper and phosphate. Shannon-wiener diversity of benthic macroinvertebrates declines with increases in phosphate (F (1, 38)=26.21, β= -0.423 ± 0.083, p<0.001) (Figure 8) and copper (F(1, 38)=2.59, β=15.441 ± 5.95, p<0.013) (Figure 9).
Variation in Amphibian and Macroinvertebrate Abundance and Diversity
We observed significant among site variation in amphibian diversity, and macroinvertebrate diversity and abundance, which appeared to correspond to changes in water chemistry and physical habitat condition thus providing support for my research hypothesis. My results are similar to other studies that demonstrated reductions in some freshwater populations as a result of anthropogenic alteration of riparian and aquatic habitat [7,37,38].
Numerous studies have shown that humans alter the structure and function of river ecosystems, such as reducing certain benthic macroinvertebrate species and reducing diversity, through their activities [39]. An investigation of the Cape River, South Africa found that spatial and temporal changes in benthic invertebrate communities were correlated with physical-chemical quality of the river. Braukmann and Böhme showed salt pollution affected benthic macroinvertebrate in the Werra River, Germany: Shannonwiener diversity was reduced between the upper and lower sections of the stream below discharges of chemical contaminants. Specifically, invertebrate diversity was negatively correlated with phosphorus, nitrate, ammonium-nitrogen, carbonate hardness, sulfate, chloride and calcium; the chemical changes were caused by agriculture and domestic wastewater. A similar spatial pattern was shown in my study as chemical concentration increased from upper to lower sites especially at site C where industrial effluents and domestic sewages discharged directly to the river. We also observed corresponding reductions in benthic invertebrate and amphibian diversity at sites C and D. Benthic macroinvertebrates are a diverse group and display variation in their tolerance to contaminants ranging from tolerant to sensitive taxa [40,41]. Macroinvertebrates diversity was significantly higher at sites A and B compared to C and D, this changed was likely a result of loss of sensitive taxa that were replaced by an increase in tolerant species. In my study, Chironomidae was more abundant in highly polluted sites (e.g., D) compared to other sites, while other taxa, such as the Caenidae, were most abundant at my reference site (A). Winner et al. described the distribution of benthic macroinvertebrates in streams dosed with copper for two and a half years; they found that choronimids were more abundant relative to other groups and thus more tolerant to a mean dose of 38 μg l-1 copper.
My modelling results provide additional evidence that changes in invertebrate community structure was partly a result of changes in water chemistry. Temperature and principal component one (PC1) which represented the aggregated chemical measures were the most significant predictors of invertebrate diversity. Copper and phosphate were important components of PC1, and they were also negatively associated with invertebrate diversity. Temperature is an important environmental variable associated with aquatic and terrestrial organisms [42,43]; most aquatic organisms are ectothermic and thus the ambient environment determines their metabolic functions. In my study, some invertebrate families (i.e., Heptageniidae, Leptophlebiidae, Caenidae and Baetidae) were found mostly at the upper stream in a temperature range of 14 to 16°C, while they were mostly absent at lower stream sites in a temperature range of 23 to 25°C; mayflies have been reported to be sensitive to increased water temperature [44].
River width was the only significant predictor of macroinvertebrate abundance; this relationship was also shown in the Langat River, Malaysia [45]. We are unsure of the mechanism(s) explaining the negative effects of river width on invertebrate abundance, but we hypothesize it was likely a result of factor(s) associated with width such as changes in water temperature or habitat damage.
Similar to invertebrates, amphibians were associated with variation in the physical and chemical properties of the river. For example, we observed 10 species at sites A and B but only 6 at sites C and D. River width and water temperature were the two predictor variables associated with amphibian abundance (width, negative slope) and diversity (temperature, negative slope). As with invertebrate abundance, changes in amphibian abundance with river width was likely a result of factors correlated with width such as habitat damage or water temperature. The negative relationship between water temperature and amphibian diversity is in agreement with other studies that have found amphibians sensitive to climatic variables. Increased water temperature can directly affect amphibians by reducing growth and survival but also can increase their susceptibility to pathogens [46].
It was my expectation that chemical properties of the river, such as chloride concentrations, would have a major impact on amphibian abundance and diversity; however, this was not the case. This result might be due to relatively low chloride concentrations (i.e., 47 mg/L) at my study sites compared to other studies [47]. Swadoski showed that, amphibian abundance and diversity were consistently low in wetlands where chloride levels exceeded 200 mg/L. In addition, the fact that changes in water chemistry were associated with changes in habitat structure and water temperature limit my ability to observe an effect of chemistry on amphibians. Furthermore, there were possibly unmeasured variables that affected amphibians. Some contaminants are associated with human waste and interfere with the hormonal system of amphibians altering sex determination, such as in early growing stages of Rana temporaria and Xenopus laevis, without inducing direct toxicological risk on species; the portion of females for both species decreased from controls to 1:12 to 1:2 mixture of sewage water with steroids and alklyphenol [12]. Therefore, if a female’s reproduction decreases as a result of exposure to hormones, it might affect the population size of that species thereby leading to changes in species composition. Overall, my results support the notion that amphibians are excellent bioindicators. First, they are sensitive to changes in the environment because their permeable skin easily absorbs chemicals from the environment [48,49] and changes in moisture. Second, they are ectothermic thus sensitive to changes in temperature [50,46]. Third, they are relatively species-rich along the Themi River exhibiting a range of life histories and vulnerability.
Habitat destruction
Amphibians and benthic macroinvertebrates of Themi River were also influenced by changes in habitat characteristics along the Themi River. These organisms require relatively undisturbed habitat to grow, survive and reproduce [51,52], Habitat destruction and fragmentation have been identified as possible causes of large-scale amphibian declines [53] and changes in benthic macroinvertebrate communities [54]. The Themi River is surrounded by thousands of household that directly depend but impact river habitat. Thus, multiple land use changes were associated with the amount of disturbed habitat, which increased at sites C and D, where we also observed sharp declines in invertebrate and amphibian diversity.
This is the first study that we are aware that assessed the human impact on chemical, physical, and biological properties of the Themi River. Moreover, there are relatively few similar studies conducted in other parts of Africa, although this continent has one of the world’s highest population growth rates, which threatens a variety of terrestrial and aquatic ecosystems [55,56]. In recent years, management of stream biodiversity in presence of human encroachment has received much consideration [57,58]. We decided to study these impacts so as to highlight the magnitude and effects of aquatic pollution, and habitat destruction to population abundance and diversity of amphibian and benthic macroinvertebrates. These results will be used as one of the major source of information that will help in decision-making toward protection of freshwater and conservation of freshwater organisms since the Themi River is an important water source for humans and wildlife.
This was an observational study, therefore we cannot infer mechanisms explaining the variation we observed. A major issue in stream monitoring studies is that structural and functional characteristics change naturally from upstream to downstream, so it is a challenge to separate natural variation in stream ecosystems with variation resulting from humans. In addition, we collected multiple samples within a transect, which may affect spatial independence. we recommend experimental studies that are based on my observational results.
We recommend the following approaches to reduce the human impact to Themi watershed: (1) We advise the municipality and associated villages to reduce human activities in and along the Themi River including the collection of building materials (stones and sand from the river bed), development of vegetable gardens close to the banks, cleaning and washing businesses and the discharge of sewage through pipes and tributaries to the main stem river and (2) the Arusha urban water and sewage authority must treat sewage water before releasing to the river. Adopting these simple measures will ensure a sustainable sou