e-ISSN: 2321-6182 p-ISSN: 2347-2332
e-ISSN: 2321-6182 p-ISSN: 2347-2332
Osvaldo R. Neto, Thais R. Casagrande, René A. Ferreira, Cristiani Bürger, Angela Malheiros, Angélica G. Couto*
Núcleo de Investigações Químico-Farmacêuticas (NIQFAR), Universidade do Vale do Itajaí (UNIVALI). Rua Uruguai, nº 458, setor E1, CEP 88302-202, Itajaí – SC, Brasil
Received: 07/03/ 2015; Accepted: 10/04/ 2015; Published: 15/4/2015
Visit for more related articles at Research & Reviews: Journal of Pharmacognosy and Phytochemistry
Campomanesia reitziana, popularly known as guabiroba in South and Southern Brazil, belongs to the Myrtaceae family. Among other species, Campomanesia xanthocarpa has been used to reduce hunger by chewing the leaves. Our study aimed to characterize Campomanesia reitziana as a pharmaceutical raw material. Fresh leaves were described by botanical analysis. After drying, the leaves were powdered and characterized by loss on drying (LD), particle size, water extractable matter (EM), total and acid insoluble ash (TA and AIA), total flavonoids (TFC) and total phenolic (TPC) contents, and thin layer chromatographic (TLC) profile. Powdered leaves were classified as coarse powder, 5.43 (%, w/w) of TA and 0.55 (%, w/w) of AIA, 23.88% (w/w) of EM, 16.30 mg/g of TFC and 2.95 mg/g of TPC. TLC revealed the presence of a major flavonoid, probably a glycosylated phenolic compound, as pointed by NMR analysis, and which need further studies for identification.
Quality control, Campomanesia reitziana, Campomanesia xanthocarpa, Flavonoids, Phenolics
Brazilian people have easy access to many types of medicinal plants, specially due to the rich biodiversity in their country. Aligning this feature with the current culture and a lack of financial resources, the use of plants for the treatment of diseases is common among this population. To obtain these herbal active compounds, the establishment of quality criteria is essential to ensure the efficiency of the final product, since, even within the same plant species, factors related to biological variability, cultivation, collection and subsequent treatment can decisively influence the characteristics of their suitability for intended use [1-3].
Each plant species has some unique characteristics, which identification should begin with larger macroscopic structures. For example, if the plant is an herb, it is observed in relation to the position of the leaves, organization of the flowers and branches, form of the stem, among others. However, the common practice in the pharmaceutical industry is to have the raw material already crushed by the plant supplier. Therefore, microscopic identification is essential for quality control of herbal drugs to prevent the adulteration of raw material [4-6].
Leaves of several species of Myrtaceae are commonly chewed to reduce hunger and promote weight loss [7]. The bark and leaves of guabiroba of Reitz are astringent and they are used to treat diarrhea and cystitis [8]. Myrtaceae is a family in which at least 132 genera and over 5600 species, including the genus Campomanesia Ruiz & Pavón which has 41 species and 32 are endemic in the Brazilian flora [9,10].
Campomanesia reitziana D. Legrand, popularly known in Brazil as ‘guabiroba’ or ‘guabiroba of Reitz’, belongs to the family Myrtaceae, and is native, endemic and distributed in South and Southern Brazil [10-12]. Campomanesia is a genus of trees and shrubs that ranges from northern Argentina to Trinidad, and from the coast of Brazil to the Andes or Peru, Ecuador, and Colombia [11].
Morphological and anatomical studies in Myrtaceae has been related [13-15]. The following characters appear to be taxonomically useful in the Myrtaceae family: epidermal common cell format, presence of dibrachiate trichomes, presence of colorless subepidermal cell layers and midrib shape [15]. In Campomanesia there are (3-)4-18 locules in the ovary, the walls of which become thickened and glandular as the fruit matures. There are several ovules per locule, but normally all, or all but one, abort. In fertile locules the seed has a very delicate, thin seed coat, but the seed is protected by the locular wall that serves as a false seed coat [11]. However, the borders between some species are poorly understood, and for this reason, Landrum (1986) mentioned the formation of three groups based primarily on characters of flowers, in which Campomanesia reitziana Legrand and Campomanesia xanthocarpa O. Berg belong to the same group named “Campomanesia xanthocarpa complex”.
Taking into account the risks of using wrong plant species in the production of herbal medicines, the importance of the diagnosis of Campomanesia reitziana is highlighted. For example, while Campomanesia reitziana D. Legrand is characterized by leaves, Campomanesia xanthocarpa (Mart.) O. Berg presents an wide morphological variation, with leaves of various sizes and textures. Campomanesia xanthocarpa occurs closest to the coast, individuals have long and more robust leaves. The leaves of Campomanesia reitziana can be bullate or not, with margin markedly denticulate, base often cordate, and glands visible only on the abaxial surface. The bracteoles can be persisted to the present fruit and edges denticulate [16].
The Myrtaceae family is characterized by the presence of essential oils and polyphenols [17]. Studies reported the presence of flavonoids in species of Campomanesia [18-20]. In Campomanesia guazumifolia, quercitrin, myricetin and 3-O-rhamnoglicosid were isolated; in Campomanesia pubescens, myricetin; and in Campomanesia xanthocarpa, miricitrina and rutin[21]. Chalcones and flavanones have also been isolated and identified in the leaves of Campomanesia adamantium species [22].
In Brazil, the current legislation to authorize the production and commercialization of herbal products requires documentation of all actions directly or indirectly involved in its production. For this purpose, the existence of official specifications of pharmaceutical raw materials, derived from medicinal plants, represents an important step towards the establishment of minimum criteria for acceptance of quality [23].
The present study aims to contribute to the quality control of Campomanesia reitziana leaves for its pharmacognostic authentication, and physical-chemical characteristics, in order to be used as used as raw materials in herbal medicine production.
Plant material
Adult leaves of Campomanesia reitziana and Campomanesia xanthocarpa were collected from some few specimens in the city of Itajaí, SC (26° 55 '45.3'' S and 48º 39' 12.8'' W), in August 2010. A voucher specimen of Campomanesia reitziana was deposited in the Barbosa Rodrigues Herbarium (Itajaí-SC), with code HBR 52578. A voucher specimen of Campomanesia xanthocarpa are deposited in the Dr Roberto Miguel Klein Herbarium (Blumenau-SC) with code FURB 11905. Leaves of Campomanesia xanthocarpa were only used for botanical analysis comparison.
Macroscopic analysis
Macroscopic analyses included visual inspection and stereomicroscopy. Phyllotaxy and leaf elements (veining, texture, color, format, apex, base, margin and indumentum) were observed, and then they were compared to the morphological patterns reported by literature [24,25].
Microscopic analysis
The leaves for anatomical study were fixed inin formalin-acetic-acid- alcohol (FAA) for 48 hours and subsequently stored in 70 º GL ethanol [26]. The slides were prepared from handmade cuts, clarified with a commercial solution of sodium hypochlorite (50%), washed with distilled water, then with acidified water, and again with distilled water. Some of the paradermic, transverse leaf blade (middle third lower sections) and petiole (middle section) were stained with Astra blue (1%) in 50 ºGL hydroethanol solution for ten minutes [27]. Microchemical reactions were applied with Sudan III to reveal lipophilic substances, and hydrochloric phloroglucinol was used to reveal the lignified elements [24]. The chemical nature of the crystals was analyzed through their solubility in acids [28]. To observe the adaxial and abaxial surfaces and stomata, paradermic sections were used. They were visualized with 40x, 100x and 400x magnification [29]. Measurements of structures were taken using a micrometer scale lamina and observations were captured in a Leica CME optical microscope.
Previous treatment of the plant material
After collection, the leaves were cleared of foreign material and placed on trays in a drying room until moisture stabilization. The dried leaves were ground to a powder in a hammer mill with 3 mm sieve.
Determination of loss on drying of fresh plant material
Samples of 12 g (n = 5) of the fresh leaves were placed in a drying room. The loss on drying (%, w/w) was controlled by the mass difference every 24 h until stabilization.
Determination of loss on drying
Samples of 1 g (n = 4) of dried and powdered leaves were analyzed, using an infrared balance at 105 °C [30].
Particle size analysis by sieving
Approximately 50 g (n = 3) of dried and powdered plant drug was passed through sieves of 2, 1, 0.850, 0.500, 0.250 and 0.150 mm for 15 minutes. Equation 1 was used to calculate the average particle diameter (Equation 1) and a histogram was plotted of particle size distribution [31].
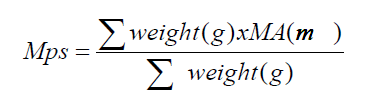 (1)
(1)
where Mps = mean particle size (mm); MA = mean aperture (mm).
Determination of total ash and insoluble ash in hydrochloric acid
These tests were performed according to the pharmacopeia procedures [30], using three samples of 3 g.
Chemical characterization by qualitative thin layer chromatography and nuclear magnetic resonance
About 1 g of dried and powdered plant, along with 20 mL of alcohol 70 ° GL were submitted to reflux for 20 minutes, pressed through Sontara® and filtered through filter paper for preparation of sample solutions. The samples were applied to silica gel GF254 sheets, using two eluent systems: 1) formic acid: acetic acid: water: ethyl acetate (11:11:27:100, v/v) [32]; and 2) ethyl acetate: acetone: water: methanol (25:8:1:3, v/v). Quercetin, rutin and isoquercitrin were used as reference substances dissolved in methanol. After drying at room temperature, the spots were detected under UV light at 365 nm, after spraying with the Natural product Reagent A, consisted by 1% (diphenylboryloxy) ethylamine (w/v) methanol solution for revelation, and polyethylene glycol 4000 0.5% (w/v) ethanolic solution for fixation.
The 1H (300 MHz) spectra of ethanol extract of Campomanesia reitziana leaves was recorded on a Varian Gemini 300 spectrometer in CD3OD. The chemical shift was expressed in ppm.
Water soluble extractives
Solutions 1:100 g of dried and powdered leaves were heated in water until boiling, for 10 minutes. Samples of 20 g (n=3) of the filtrate were dried and desiccated at 100 ± 5 °C. The extracted matter was calculated by the percentage ratio between the dry residue and the raw material [33], using equation 2.
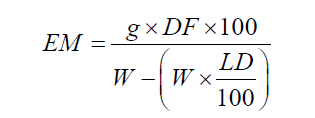 (2)
(2)
where EM = extracted matter (%, w/w); g = mass (g) of dry residue; DF = dilution factor (5); W = mass (g) of the sample and LD = loss on drying of the sample (%, w/w).
Total phenolic content
The sample was evaluated by spectrophotometry (UV-vis) with some modifications [34].
Preparation of calibration curve: The calibration curve at 750 nm was obtained from aqueous stock solutions of gallic acid (5.0 to 500 mg/mL) after 60 min of reaction with 0.5 mL of Folin-Ciocalteau reagent, 10 mL of 10% sodium carbonate in the proportion of 0.1:25 (stock solution: water, v/v).
Sample preparation
500 mg of raw material was extracted with 100 mL of ethyl alcohol by sonication for 10 minutes. Absorbance was read at 750 nm after 60 min of reaction with 0.5 mL of Folin-Ciocalteau reagent, and 10 mL of 10% sodium carbonate at a proportion of 0.1:25 (sample solution: water, v/v). The total phenolic content was calculated as gallic acid equivalent, using equation 3:
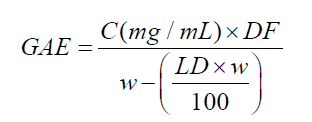 (3)
(3)
where GAE = gallic acid equivalent (mg/g), C = gallic acid concentration in the sample solution (mg/mL), calculated by correspondent calibration curve equation, DF = dilution factor (25000), w = mass of dried and powered leaves (g), LD = loss on drying of dried and powered leaves (%).
Total flavonoids content
The sample was evaluated by spectrophotometry (UV-vis) with some modifications [35].
Preparation of the calibration curve: The calibration curve at 415 nm was obtained from ethanol solutions of quercetin (2.5 to 25 μg/mL) after 60 min of reaction with AlCl3 10% solution. For each concentration, a comparative solution without AlCl3 was prepared.
Sample preparation
1.5 to 5.0 g of raw material was extracted with ethanol 70% (v/v) by sonication for 10 minutes, to determine the ideal mass of sample for this test. The extraction was made in duplicate. Aliquots of 500 μL of each extract and reagents (200 μL of Sodium acetate 1 N, 500 μL of AlCl3 10% solution) were diluted to a final volume of 5 mL. Further reaction was produced as described for the calibration curve. The total flavonoid content was calculated as quercetin equivalent, using equation 4.
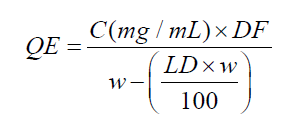 (4)
(4)
where QE = quercetin equivalent (mg/g), C = concentration of quercetin in the sample solution (μg/mL), calculated by the equation of the quercetin calibration curve, DF = dilution factor (1000); w = mass of the drug plant (g), LD = loss on drying of dried leaves (%).
Macroscopic analysis showed that Campomanesia reitziana has the following characteristics: simple leaves, with opposite phyllotaxy; lamina chartaceous, oblong, glabrous on both surfaces; apex acute; base rounded or rare acute; margin denticulate; and venation pinnate (Figure 1). The midvein and secondary veins are grooved or impressed on the adaxial surface, and brochidodromous venation is observed. The petiole is short or very short (1 – 5 mm long) and varies from straight to channeled. The leaves of Campomanesia reitziana have some morphological similarities with other species of the Myrtaceae family, usually simple leaves, opposite phyllotaxy and evergreen coloration [36]. Campomanesia reitziana leaf morphology was similar to other studies [37,38], particularly in terms of leaf composition, color, consistency, edges, contours and petioles.
In regard to the microscopic characters, we obtained the following description: asymmetrical heterogeneous mesophyll (dorsiventral), with significant presence of idioblasts containing prismatic crystals (calcium oxalate), large in size (A, B) and secretory cavities (A), epidermal cells with sinuous contour (C); uniseriate epidermis in the cross section of adaxial surface, and anomocytic stomata (D), and presence of glandular (E) and non-glandular multicellular trichomes (F) in the front view of abaxial surface. In cross section, the midvein (G) and petiole (H) have straight adaxial surface and concave abaxial surface. Both have a vascular bundle sclerenchyma surrounded by crystals of calcium oxalate and oil glands in the parenchyma. In addition, there were trichomes in the epidermis (Figure 2).
The leaves of Campomanesia reitziana have some anatomical similarities with other medicinal species of the Myrtaceae family, such as a dorsiventral mesophyll, presence of calcium oxalate crystals, secretory cavities, and non-glandular trichomes [39], as well as the presence of secretory cavities of essential oil in their vegetative organs [36].
The mesophyll of species Campomanesia xantocarpa was characterized by the presence of fine prismatic crystals in the center, uniseriate epidermis, oil glands, palisade parenchyma with one or two cell layers, spongy parenchyma of approximately 6 to 8 cell layers. The midvein region was characterized by a straight adaxial and rounded abaxial surfaces. Trichomes were observed on the adaxial surface. In the abaxial and lateral face, unicellular non-glandular trichomes were observed. Petiole was characterized by a large amount of prismatic crystals and few tector trichomes.
Comparatively, in the species Campomanesia reitziana, long unicellular non-glandular trichomes, with smooth cuticle, were observed in the midvein. The petiole was characterized by slightly concave adaxial and convex abaxial surfaces. A large amount of prismatic crystals was observed, as well as a single central vascular bundle and greater occurrence of non-glandular trichomes on the epidermal region, as an important feature in differentiating the two species.
The loss on drying control of the fresh leaves showed a moisture equilibrium at 49.78 ± 0.90 (%, w/w) in 24 h, with 8.61 ± 0.46 (%, w/w) of final moisture. This value belongs to the acceptable range of moisture (8-14 %, w/w) [30]. After milling, the moisture remained acceptable and constant, at 8.69 ± 0.53 (%, w/w), as well as favorable physical characteristics of the starting material. In terms of production, this behavior is highly preferable, as it demands less time and energy, demonstrating that the preliminary operations were effective.
The size distribution of the raw material (Figure 3) shows that 75.15% of the particles are smaller than 0.850 and greater than 0.250 mm. According to the Brazilian Pharmacopeia [30], powder with these characteristics is classified as coarse, as the particles are smaller than 1.7 mm and a minimum of 60% are greater than 355 μm. The mean particle size of dried and powdered leaves was 0.493 ± 0.006 mm.
The samples had a mean of 5.43 ± 0.0001 (%, w/w) for total ash and 0.55 ± 0.0001 (%, w/w) of acid insoluble ashes. The determination of ash content allows the verification of non-volatile inorganic impurities that may be present as contaminants [40]. Acid insoluble ashes test would allow the verification of contaminants such as dirt or sand residue, most frequently observed in the roots [40]. The low values of impurities found would be related to Campomanesia reitziana be a high tree, with leaves without contact with the soils contaminants.
In regard to chemical quality control for materials for which there is no chemical or biological assay, the water content of extractable matter is a technique that determines the amount of active ingredients extracted with solvents from a certain amount of medicinal plant material is used for [41]. In Campomanesia reitziana the water extractable matter of leaves was 23.88 ± 1.92 (%, w/w).
The total phenolic content was expressed as gallic acid equivalent. As result, it was found 16.30 ± 1.48 mg/g, using the equation obtained from the calibration curve of gallic acid solution (y = 0.0988x + 0.0012, r2 = 0.9949).
The flavonoid content was expressed as quercetin equivalent. The best amount of sample to determine the flavonoid content was 2.5 g, after that the solvent starts saturating and the absorbance values tend to stabilize. Then, the EQ was 2.947 mg/g, using the equation obtained from calibration curve of quercetin solution (y = 0.0153x + 0.0153, r2 = 0.9959) (Figure 4).
The ethanol (70 ºGL) extract of Campomanesia reitziana leaves were also characterized by thin layer chromatography. The system eluted with ethyl acetate: acetone: water: methanol (25:8:1:3, v/v), showed a unique orange spot with Rf 0.6, suggesting the presence of a major flavonoid, since it was revealed with Natural product Reagent A, which is known by its flavonoid specificity, providing yellow-orange or yellow-green spots, characteristic of flavonoids glycosides [32]. However, none of the flavonoids commonly found in another species from the same genus, such as rutin, quercetin nor isoquercitrin was identified by TLC. NMR analysis of the sample of this extract, present signs related to hydrogens of aromatic rings (6-8 ppm) and many hydrogens near from oxygens (3-6 ppm) were observed, which could strengthen the presence of glycoside units, confirming the presence of polar phenolic/ flavonoid compounds. This finding is in accordance with the phenolic and flavonoid contents quantified in this extract. Other signals below 2.5 ppm indicate hydrocarbon hydrogens.
In conclusion, this work provides useful information on macro and micro characterization of plant material, like the presence of non-glandular thricomes on epidermal region as the only evidence found to discriminate Campomanesia reitziana and Campomanesia xanthocarpa, as well as on chemical characterization, never studied before, which can help the quality control parameters for a raw material intended to be used by different fields of industry of herbal natural products.
The authors thank for the financial support and scholarship granted for Thais R. Casagrande by local institutional scholarships ProBIC/ UNIVALI.