ISSN: 2347-7830
ISSN: 2347-7830
Chirukuri Rajasekharam and Atmakuru Ramesh*
Department of Analytical Chemistry, International Institute of Biotechnology and Toxicology (IIBAT), Padappai, Kanchipuram District - 601301, Tamil Nadu, India
Received: 10 November 2013 Accepted: 28 February 2015 Published: 30 December 2013
Visit for more related articles at Research & Reviews: Journal of Ecology and Environmental Sciences
Halosulfuron-methyl is a new, low rate, sulfonylurea herbicide that is being promoted for annual broadleaf and gramineal weed control; however, there is a lack of published information on its behavior in soils. The adsorption and degradation of Halosulfuron-methyl by four soils were measured using a batch equilibrium technique. The soil sorption coefficient Kf, the soil organic carbon sorption coefficient Koc and Herbicide degradation DT50 are the basic parameters used for describing the environmental fate of the herbicide. The results showed that the Freundlich equation fitted its adsorption well, and the Frendlich constant value (Kf) ranged from 1.1-6.1. Soil organic carbon coefficient Koc ranged from 105-173. Soil pH, organic matter (OM), and clay content were the main factors influencing its adsorption. Adsorption was negatively correlated with pH 6.0-8.0 while positively correlated with OM and clay content. Persistence of Halosulfuron-methyl conducted in all the 4 kinds of soil shows the DT50 at 8.1 to 10.9 days. The sorption coefficient (Koc) and half-life (DT50) were determined by combining the values of Koc and DT50, and the mobility of residues of Halosulfuron-methyl in soil was calculated using GUS (Ground water Ubiquity Score) equation. The GUS values were found to be less than 1.9 in all the types of soils studied.
Adsorption, Degradation, GUS, Halosulfuron-methyl
Sorption is an important process in determining the fate and distribution of herbicides in environmental soil and water, thereby determining the amount of herbicide that can reach the target and the amounts that degraded and leached through the soil. The persistence and sorption properties of the compound will vary based on physical, chemical properties, texture and organic carbon content of the soils. The degradation of residues of isoproturon is significantly influenced by organic carbon, pH of the soil, the degradation rate varied based on the soil properties when incubated at 15°C [14]. The degradation of acidic and basic herbicides was significantly affected due to presence of organic carbon content, the pH influenced based on the degradation mode [9]. Prosulfuron was highly influenced by the pH of the soil, and was found highly stable in soils having basic pH [11]. The adsorption of acidic and basic herbicide was negatively influenced due to soil pH, and positively by organic carbon content [8]. The adsorption of aminocyclopyrachlor was found to be directly proportional to the organic carbon content in the soil [12]. Sorption of endosulfan, Methiopyrsulfuron and sulcotrione and its hydrolysis products were greatly influenced due to clay content and pH of the soil was reported in various literatures [10, 3, 7]. The adsorption of diuron [15] and monosulfuron [17] was found to be high at a lower soil pH. Batch sorption study with eleven pesticides in thirteen types of soil showed that the adsorption coefficient was influenced by organic matter, salinity, pH, cation exchange capacity, soil complex saturation and clay content of the soil, and the above parameters varied based on the compound nature also soil pH, organic matter (OM), and clay content were the main factors influencing its adsorption and Desorption [13]. The adsorption and Desorption of Monosulfuron-ester were negatively co-related with pH 4.0-8.0 while positively correlated with soil organic matter and clay content [4]. In the adsorption-desorption of phenylurea herbicides, the organic carbon was the main factor affecting urea sorption [5].The carbofuron adsorption capacity was significantly higher in the soil with high organic matter [1]. The adsorption/Desorption of butachlor, myclobutanil and chloropyrifos were mainly controlled by soil organic matter (OM) and octanol/water-partitioning coefficient content [16]. Atrazine, isoproturon and trifluralin adsorption were correlated to soil organic matter content bentazone adsorption was governed by soil pH while insignificant effect has been shewn in the case of 2,4-D [2].
From the literature survey, it was observed that no information has been published concerning the fate of Halosulfuron-methyl in soil. Halosulfuron-methyl is a selective, systemic post emergence herbicide used to control annual broad laved weeds and nutsedge in maize, sugar cane and paddy field. The herbicide, used in the paddy field under flooded condition, can contaminate the ground water through leaching or runoff. The study was conducted to evaluate the risk of contamination of Halosulfuron-methyl to water by determining its sorption characteristics and dissipation behavior in different Indian soils having different textural, physical and chemical properties and to correlate the factors influencing the leaching, degradation and sorption of Halosulfuron-methyl.
Halosulfuron-methyl (purity 99.9%), reference analytical standard was pursed from Sigma-aldrich (USA). HPLC grade Acetonitrile, Ortho–phosphoric acid, Magnesium chloride, Potassium chloride, Ammonium chloride, Potassium dihydrogen phosphate and Calcium chloride were all GR grade obtained from Merck India limited. Distilled water was purified by using the Milli-Q Plus apparatus (Millipore, Bedford, MA, USA).
Soil (0 to ≤ 20 cm depth) was collected from agricultural fields of different states in India; there was no previous history of Halosulfuron-methyl herbicide application. The different types of collected soils were sieved using a 2 mm diameter sieve and analyzed according to standard protocol to characterize physical and chemical properties. Soil texture was determined by using the international pipette method, Organic carbon was estimated according to the Walkley, and black and soil pH was measured in a 1:2 (w/w) soil /deionized water mixture.
A laboratory study was conducted to determine the fate of Halosulfuron-methyl in the four different kinds of soil collected from the locations: Kerala, Karnataka, Punjab and Andhra Pradesh. The physical and chemical properties viz., pH, conductivity, organic carbon content, cation exchange capacity and texture were determined. Soil samples collected from the different states in india are sieved through a 2 mm sieve and quantitatively transferred in to the vials. The soil samples were exposed to sunlight with one third content of its moisture holding capacity, acclimatized for a period of three days before fortification. Following the acclimatization, the soil samples were fortified at 0.1 mg/kg of Halosulfuron-methyl and exposed to direct sunlight. On pre-determined intervals, duplicate soil samples were removed and extracted for the determination of residues.
The soils were extracted using the mixture of 20 mL of Acetonitrile: water (90/10 v/v) and tumbled for a period of four hours using end over end mechanical shaker. After tumbling the solvent layer was separated by using centrifuge, the extraction procedure was repeated with another 20 mL of extraction solvent. The extracts were pooled together and concentrated under the stream of nitrogen at 30°C. The residues were reconstituted using 5 mL of acetonitrile for the HPLC analysis.
The batch equilibrium technique was used to determine the soil adsorption constants of Halosulfuron-methyl in four different types of soil. A preliminary study was conducted to determine the soil/solution ratio, the equilibrium time for adsorption, the adsorption of the test substance on the surface of the test vessels and the stability of the test substance during the equilibration process. Before initiation of the experiment, the soil samples were sterilized by drying in the oven at 105°C for a period of 6 hours. Sterilization process was performed to restrain (to prevent the biodegradation of Halosulfuron-methyl) microbial degradation, the 100 mL polypropylene centrifuge tubes were filled with 5 g of sterile soil. An aliquot each of 5, 10, 25 and 50 mL of 10 mM CaCl2 solution was added to each vial and equilibrated for four hours at room temperature. To the test vessels, a 5, 10, 25 and 50 mL of 5 μg/mL dosing solution was added to get the soil solution ratio 1:2, 1:4, 1:10 and 1:20, and kept in a horizontal shaker at 50-60 rpm. On pre-determined sampling occasions (2, 4, 8, 16 and 24 hours), triplicate samples were removed for the quantification of residues. After the adsorption process described above, a 5 ml of the supernatant solution was withdrawn and the amount of adsorbed was calculated. The remaining slurry was again brought to 10 mL by the addition of 5 mL of 0.01 M CaCl2, equilibrated for 24 hr. these steps were repeated three times consecutively. Based on the preliminary results, sorption study was conducted with soil solution ratio 1: 2 (5 g soil + 5mL of 0.01M CaCl2 + 5 mL of respective concentration of Halosulfuron-methyl in 0.01M CaCl2) and compound concentrations are 0.1, 0.5, 1.0, 2.0 and 5.0 μg/mL, having equilibration time 16 hours and triplicate samples of each soil and each concentration were used to determine the sorption study. To check the interferences in solution and stability of the test item, one rotating soil free control, one static soil free control and standard free soil control were analyzed simultaneously. All vials except the static soil free control were tumbled on a rotator for 16 hours at 50-60 RPM at room temperature. The vials were removed from the rotator and centrifuged for 5 minutes at approximately 2000 RPM in a cooling centrifuge at 5 – 10°C. After centrifugation, the supernatant was filtered using 0.45 μ PTFE membrane filter and transferred to HPLC vials for analysis.
An Agilent® 1200 series High Performance Liquid Chromatograph equipped with Diode array detector was used for the quantification of residue. The detector wavelength was set at 240 nm. The separation of herbicide residues was carried out using the Zorbax® column SB-C18 (3.5 μm particle size, 4.6 mm i.d. and 75 mm length). The mobile phase used was (ACN: 0.1% H3PO4). The flow was programmed and a 1.0 ml per minute flow rate was set. The injection volume 50 μl was set for standard and sample. Halosulfuron-methyl was eluted at 8.0 minutes.
Specificity, linearity, assay accuracy and precision were done to validate the method. The specificity of the method was confirmed by injecting the control soil extracts, mobile phase, acetonitrile, extraction solvent and diluting solvents, buffer solutions. Different known concentrations 5.0, 2.5, 1.25, 0.1, 0.05 and 0.01 mg L-1 of Halosulfuron-methyl was injected for linearity. Mobile phase was used for the preparation of calibration solutions by diluting the stock solution. The peak area was measured after injecting each calibration solution. Correlation coefficient was calculated from the plot against concentration of the standards versus area observed. Based on the signal to noise ratio of 3:1, the limit of detection of the instrument was established. Recoveries of residues in four different textured soils were studied by fortifying known concentration 0.02 (LOQ) and 0.2 mg kg-1 (10 x LOQ level) of standard in each soil. Five replicate determinations were used to check the precision of the method. The samples were homogenized using homogenizer after fortification of standard, extracted and analysed for the residues of Halosulfuron-methyl by HPLC-DAD method. Based on the recovery study, the limit of quantification was established as 0.02 mg kg-1. Repeatability of the method showed acceptable RSD% according to the ‘Hurwitz equation’ (1).
RSD% < 2 (1-0.5 logC) x 0.67
Where C is the concentration of the analyte expressed in percentage. The maximum acceptable RSD% calculated based on the above equation for the analyte concentrations 0.2 mg Kg-1 was 12.89% and for 0.02 mg Kg-1 18.10%.
The amount of Halosulfuron-methyl adsorbed after equilibrium was calculated according to the difference between the initial and final equilibrium solution concentrations by the following equation (2)
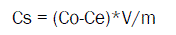
Where, Cs (mg/kg) is the amount of Halosulfuron-methyl adsorbed by soil and Co (mg/L) is the initial and equilibrium aqueous concentration, respectively. V (mL) is the volume of the solution and m is the mass of the soil.
Adsorption and Desorption were described by the linearized form of the Frendlich equation (3)
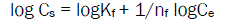
Where, Kf is the adsorption coefficient characterizing the adsorption-desorption capacity, and nf is the Freundlich equation exponent related to adsorption intensity that is used as an indicator of the adsorption isotherm nonlinearity. Kf-ads is the adsorption coefficient and Kf-des is the desorption coefficient of the Frendlich equation.
The OM normalized adsorption constant (Kom) was calculated by normalizing Kf-ads to the fraction of OM according to equation (4)
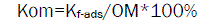
The free energy of adsorption of the herbicide in soil is calculated using the thermodynamic equation (5)
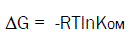
Where, ΔG (kj/mol) is the free energy of adsorption, R (8.314*10-3 kj/(K.mol)) is the mol gas constant, mol gas constant, and T (K) is the absolute temperature.
Figure 1 shows the adsorption kinetics after shaking four soils with 5 μg/mL of Halosulfuron-methyl in 0.01M aqueous solution. The rapid adsorption phase took place within 5 hours after shaking. After 5 hours, the herbicide concentration in solution varied with small changes, the adsorption phase slowed. This study found that adsorption equilibrium was reached 8 hours in all soils tested. Moreover, the amount adsorbed by soil remained steady. Further decreases in the Halosulfuron-methyl concentration in the supernatant after 24 hr were not significant. For laboratory convenience, 16 hr was taken as the equilibrium time.
The Freundlich adsorption isotherms are shown in Figure 2. The values for the adsorption coefficient (Kf), were calculated for all the test soils. The Freundlich constant values Kf ranged from 1.1 to 6.1 mL/g and sorption isotherms were non linear with 1/n values < 1 among the soils tested in 0.01M CaCl2 as a background electrolyte. The adsorption coefficients were normalized to the organic carbon contents for each soil to calculate the soil sorption coefficients (Koc). The (Koc) values ranged from 105-173 among the soils tested. The adsorption isotherm of Halosulfuron-methyl dependant on soil types, and the order of Kf-ads was Punjab > Kerala > Karnata > Andhrapradesh. The Kf and Koc values were low for the soils having low organic carbon and clay content, adsorption of Halosulfuron-methyl was high for the soils having higher organic carbon and clay content, as confirmed from the Kf and Koc values. The comparison between pH of the soil, distribution coefficient, organic carbon content and half life was presented in Figure 3, observed that these parameters found directly related each other. The sorption coefficient found directly proportional to the soils having higher organic carbon and lower half life. Results are presented in Table 1.
The ΔG value of 40kj/mol was considered as a threshold for identifying the physical and chemical mechanisms of adsorption, and physical adsorption mainly was involved below threshold. The ΔG values of four soils at 25°C ranged from -4.96 to -4.42 kJ/mol, the ΔG of halosulfuron-methyl in four soils was less than 40 kj/mol, indicating that the adsorption of halosulfuron-methyl by four soils was mainly physical process. The adsorption of halosulfuron-methyl by soils also was a spontaneous process for negative value of ΔG.
The soils collected from the different locations around India had the following compositions of Clay (10- 38%), Slit (8-36%), Sand (26-66%) and organic carbon (0.6-3.4%) and the pH was found in the range 5.0 – 7.8. Dissipation of Halosulfuron-methyl followed first order kinetics in all the tested soil. From the dissipation study it was observed that the degradation of residues was not much significantly influenced by the soil organic carbon and pH. Dissipation data in soils is presented in Figure 4.
Environmental effect of Halosulfuron-methyl was studies using the Gustafson equation [6]. The leaching potential of the residues can be determined from the formula, higher the GUS value indicates higher risk of contamination of ground water through leaching of residues from surface. Sorption coefficient (Koc) and Half life (DT50) were used to obtain GUS values. These values are numerical and obtain GUS values following formula
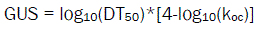
Where,
DT50 - Time taken for the test item to degrade half of its concentration
Koc - soil sorption coefficient
From half life and Koc, the GUS values calculated for each soil are in the range 1.3 to 1.9. The value indicates the leaching potential of the herbicide residue was found to low leachable through the soil. A comparative results stating the physical, chemical properties of soil, half life of Halosulfuron-methyl in soil, Koc value for each soil and GUS value calculated are presented in Table1.
Dissipation of Halosulfuron-methyl followed first order kinetics in all the tested soil. From the dissipation study it was observed that the degradation of residues was not much significantly influenced by the soil organic carbon and pH. By using the batch equilibrium experiments, the sorption characteristics of Halosulfuron-methyl were determined in four soil types. The Frendlich constant (Kf-ads) values ranged from 1.1 to 6.1 mL/g, and soil clay content, OM were the main factors affecting Halosulfuron-methyl adsorption. Adsorption was positively influenced by organic carbon content and clay content. From the analytical results, it was concluded that the Halosulfuronmethyl might be low leachable with the soils studied. The ΔG values of four soils ranged from -4.96 to -4.42 kJ/mol, the ΔG of Halosulfuron-methyl in the four soils was less than 40 kJ/mol. The negative value of ?G indicates that the adsorption of Halosulfuron-methyl was mainly physical process and also a spontaneous process.
The authors thank the management of IIBAT for providing necessary facilities to conduct the research