e-ISSN No.:2581-3897
e-ISSN No.:2581-3897
Ariane de Oliveira Troguilho1, Fernando Wiecheteck de Souza3, Giancarlo Riger3, Hayane Ramires dos Santos Souza1, Fernando Mateus Fappi3, Alanis Carolina Arias3, Luis Felipe de Sousa Lima1, Anna Julia Brandão Paes de Barros1, Alison Ferreira1, Roberto Lopes de Souza1,2*
1Department of Veterinary Medicine, Federal University of Mato Grosso (UFMT), Cuiaba, Mato Grosso, Brazil
2Department of Veterinary Medicine, Federal University of Alagoas (UFAL), Maceio, Alagoas, Brazil
3Department of Veterinary Medicine, Marechal Rondon College (FARON), Vilhena, Rondonia, Brazil
Received: 09-Jun-2025, Manuscript No. JVS-25-166584; Editor assigned: 11-Jun-2025, PreQC No. JVS-25-166584 (PQ); Reviewed: 20-Jun-2025, QC No. JVS-25-166584; Revised: 25-Jun-2025, Manuscript No. JVS-25-166584 (R); Published: 30-Jun-2025, DOI: 10.4172/2581-3897.9.02.001.
Citation: Troguilho ADO, et al. Role of Amlodipine and Hydralazine in the Management of Chronic Mitral Valve Degeneration. J Vet Sci. 2025;09:001.
Copyright: © 2025 Troguilho ADO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Research & Reviews: Journal of Veterinary Sciences
Objective: Domestic dogs (Canis lupus familiaris) may exhibit anatomical variations of the extrahepatic biliary system (hepatic ducts, cystic duct, and common bile duct). These anatomical variations directly impact the choice of surgical technique for managing pre-existing pathology in the canine extrahepatic biliary system. This study aimed to detail the anatomical features of the extrahepatic conformation and hepatic duct branches, focusing on determining an average size for the cystic duct (D-cis) across samples.
Methodology: Forty-one livers from mixed-breed dogs, over 12 months of age and weighing between 5 kg and 30 kg, with no history of hepatic injury, euthanized or deceased naturally, were analyzed. Measurements of length and width (cm) of the gallbladder (Vb), cystic duct, common bile duct (D-Com), and biliary tree lobar patterns were collected. Radiopaque dye was injected followed by a corrosion casting technique to enable both radiographic and macroscopic visualization of the biliary tree structures.
Results: Different anatomical conformations and sizes of the D-cis, D-Com, and Vb were observed, with a linear relationship between body weight and D-cis length.
Conclusion: This study is the first to define an average size of the cystic duct in dogs, allowing for its estimation based on the animal’s weight.
Cystic duct; Canine; Anatomical model; Cholecystectomy; Gallbladder
Diseases affecting the biliary system in dogs include cholangiocarcinomas, cholecystitis, cholangitis, cholelithiasis and biliary obstructions. These conditions are relatively common and often carry a poor prognosis, requiring surgical intervention [1-3]. The extrahepatic biliary system is composed of the hepatic ducts, cystic duct, common bile duct, and the gallbladder [4].
In dogs, bile flows from the bile canaliculi into the interlobular ducts and then into the lobar ducts before exiting the liver. The lobar ducts drain into the hepatic ducts, which in turn carry bile into the common bile duct [5]. Bile is then transported through the common bile duct to the duodenum and is stored and concentrated in the gallbladder. The gallbladder is pear-shaped and, in medium-sized dogs, stores approximately 15 ml of bile [4]. The common bile duct is about 5 cm long and 2.5 mm in diameter and drains into the duodenum approximately 1.5 cm to 6.0 cm distal to the pylorus at the major duodenal papilla [6].
Anatomical variations in the formation of the biliary ducts are common [7]. The cystic duct extends from the neck of the gallbladder to its junction with the first hepatic tributary. From this point to its opening into the duodenum, it is termed the common bile duct [4]. The cystic duct exhibits the highest variability in terms of length, diameter, and point of entry. In contrast, the main bile duct divided into the common hepatic duct and the choledochus shows fewer but significant variations.
Approximately 10% of dogs present variations in the lobar drainage of the hepatic ducts and common bile duct. These anatomical variations can lead to erroneous surgical decisions regarding cholecystectomy, cholecystoduodenostomy and cholecystojejunostomy, particularly in cases of intra-luminal or extra-luminal obstructions of the extrahepatic biliary system [8].
Many details of the spatial geometry of the biliary bed remain unexplained or uncharacterized. Conditions caused or associated with common bile duct ligation, such as ductular reaction and the connection of congested bile ducts with interstitial and lymphatic pathways, remain poorly understood and require further investigation. Given the frequency of anatomical variations in the biliary ducts, a detailed understanding of the spatial architecture of the biliary bed is crucial [9].
Cholecystectomy is the most commonly performed extrahepatic surgery in small animals. Laparoscopic access is well established and widely used in human medicine, and it is increasingly being adopted in veterinary medicine, with good prospects for becoming a routine procedure in small animal surgery. In humans, iatrogenic biliary duct injuries (BDIs) are potentially fatal, with high morbidity and mortality rates. They occur mainly as complications of cholecystectomy, with an average incidence of 3-6 cases per 1000 procedures [10].
The objective of this study was to detail the anatomical aspects of the extrahepatic biliary system (hepatic ducts, cystic duct, and common bile duct) in domestic dogs, and to characterize possible differences in the biliary tree drainage patterns.
This study was conducted in five distinct stages: The first consisted of the reception, storage and freezing of the cadavers; the second involved specimen preparation, which included the resection of the liver along with the distal portion of the duodenum; the third stage encompassed the acquisition of radiographic images and measurement of the proposed distances; the fourth stage included the statistical analysis of all indexed data, evaluating ductal distances and the biliary drainage patterns of the hepatic lobes. Subsequently, a corrosion casting technique was performed to produce macroscopic anatomical models of the extrahepatic anatomy.
Reception of cadavers
A total of forty-one mixed-breed dog cadavers, aged over 12 months and weighing between 1 kg and 30 kg, were received, having been euthanized or deceased naturally. These dogs were voluntarily donated by their owners, who were informed of and signed a cadaver donation consent form for research purposes. The cadavers were stored frozen. For specimen preparation, they underwent a natural thawing process by submersion in water for 12 hours.
Specimen preparation
Preparation of anatomical specimens
After thawing, each cadaver was positioned individually in dorsal recumbency in a surgical trough. A pre-retro-umbilical median celiotomy combined with a right hypochondriac paracostal celiotomy was then performed. Following exposure of the abdominal cavity, the liver was resected en bloc along with the descending portion of the duodenum containing the major duodenal papilla (Pdm).
The anatomical specimens were rinsed under running water. A duodenotomy was then performed to locate the major duodenal papilla, which was accessed using an 18G catheter. A 0.9% NaCl solution was injected retrogradely to flush the entire biliary tree, ensuring better penetration of the radiographic contrast solution. Through the major duodenal papilla, 10 ml of barium sulfate was retrogradely injected in specimens up to 10 kg, and 20 ml in specimens up to 30 kg, respecting the speciesâ?? bile secretion rate. A ligature was then placed at the major duodenal papilla to prevent leakage of the injected content.
Radiographic imaging and data collection
Each specimen was individually radiographed in a caudocranial view using a PXM 20BT X-ray emitter calibrated at 40 kV and 2.9 mAs. All images were digitally processed and the following dimensions were measured using the distance measurement function in the ZVIEW Digital Radiography System (PIXXGEN): Gallbladder width (LVb), gallbladder length (CVb), distance from the gallbladder to the first hepatic duct (Vb-IDH, representing the cystic duct), cystic duct width (LDCis), distance from the first hepatic duct to the major duodenal papilla (IDH-Pdm, representing the common bile duct), and common bile duct width (LDCom). These data were recorded in an Excel spreadsheet along with weight and age, which were then compared and subjected to statistical analysis.
Statistical analysis
For statistical analysis, ductal lengths were measured and compared with other variables that might influence them. To determine whether one variable is associated with another, we calculated the correlation between them, defined by:
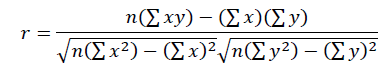
where nn is the sample size. The coefficient of determination ( 2 2) indicates the proportion of the variation in y that can be explained by the linear relationship between x and y.
To complement the statistical analysis, mean duct lengths were calculated using the equation:
xˉ=Σxinxˉ=nΣxi…………………………………(Equation 1)
where xixi represents each observation and nn is the total number of observations.
Given the expected variations in duct lengths, the standard deviation was calculated using the equation:
s=Σ(xi−xˉ)2n−1s=n−1Σ(xi−xˉ)2……………………….(Equation 2)
Corrosion casting technique
After the radiographic images were obtained, the ligatures of the major duodenal papilla (Pdm) were sectioned to remove the barium sulfate contrast. The Pdm was then recatheterized, and a 0.9% NaCl solution was retrogradely and copiously injected to flush the biliary tree and remove any residual contrast before resin injection.
Subsequently, an autopolymerizing acrylic resin solution was prepared using green Xadrez® dye with the following proportions: 15 ml of autopolymerizing acrylic liquid, 11 grams of acrylic resin powder, and 15 drops of Xadrez® dye, mixed until a smooth, fluid consistency was achieved. This solution was aspirated into a 20 ml syringe and injected retrogradely through the Pdm. The specimen was then left at room temperature until the resin polymerized.
After hardening, the sample was immersed in a corrosion solution composed of sodium hydroxide and water, left for an average of 72 hours with the solution changed every 24 hours. After complete corrosion, the anatomical specimen was rinsed thoroughly with water.
The barium sulfate contrast injected via the major duodenal papilla (Pdm) proved efficient in highlighting, through radiopacity, the anatomical conformation of the biliary tree, including the cystic duct (D-cis), common bile duct (D-Com), gallbladder (Vb), and the distribution and branching of the hepatic ducts (D-hep). This contrast agent, together with the radiographic images, enabled the measurement of the branching and conformations of the biliary tree as shown in Figure 1.
The extrahepatic and intrahepatic branching of the common bile duct and hepatic ducts appeared distinct in each sample, and they could be identified in both the contrast-enhanced radiographic images and the schematic diagram. Additionally, two distinct branching patterns originating from the common bile duct were observed: the "magistral" pattern was present in 65.9% of the samples, while the "dendritic" pattern was seen in 34.1%. In the dendritic group, some samples exhibited double and triple hepatic duct branching as shown in Figure 2.
a) CRCL projection radiographic image highlighting magistral extrahepatic structures stained with barium sulfate.
b) Anatomical specimen prepared using the corrosion casting technique from a magistral sample.
c) Schematic diagram illustrating the magistral branching.
Corrosion casting proved to be extremely effective and educational for the anatomical study of the structures in question, as it macroscopically characterized the common bile duct (D-Com), cystic duct (D-cis), gallbladder (Vb), as well as the dendritic and magistral branches of the hepatic ducts (D-hep).
In the radiographic measurements, different sizes of D-cis, D-Com, and Vb were identified. Data analysis revealed a linear relationship between the length of the D-cis and the animalsâ?? weight. In Figure 3, we present the data of weight versus D-cis length, which demonstrate a linear trend with r2 = 92.32% and an angular coefficient of 0.0915. Additionally, it was observed that animals weighing up to 20 kg had a mean cystic duct length of x = 0.937 cm and a standard deviation of s = 0.375 cm, while animals weighing more than 20 kg had a mean length of x = 2.16 cm and a standard deviation of s = 0.72 cm.
The animals investigated exhibited different types of gallbladders, classified as either Magistral or Dendritic, with the results presented in Figure 3. The r2 values were 0.9353 and 0.9513, respectively. This indicates that both types exhibited a strong linear correlation, with only a small variation in the angular coefficient of 0.017.
Discussion
The predominant complications in cholecystectomies in both dogs and humans were bile leaks due to failed cystic duct ligations (3% to 8%). In humans, this occurs in 6% of cases, in agreement with another study reporting a 3.4% bile leakage rate into the abdominal cavity [11, 12]. An important aspect to consider is the anatomical variation of the biliary ducts. A short cystic duct or a sessile gallbladder draining directly into the common hepatic duct or right hepatic duct can lead to misinterpretations in identifying the cystic duct, making its surgical anatomical identification crucial, though sometimes impossible [13].
The biliary tree is complex because the canine liver is divided into six lobes by deep fissures. A radiographic study showed that 30% of dogs had three hepatic ducts and 70% had four hepatic ducts [8]. In the present study, using contrast radiography, 46.34% of the samples had four main hepatic ducts, 26.82% had five primary branches, 19.51% had three branches and 7.32% had two primary ductal branches.
In this study, using corrosion casting with autopolymerizing acrylic resin and green Xadrezî dye, combined with a sodium hydroxide and water solution, extrahepatic structures were efficiently delineated, aiding the anatomical identification of the D-cis. Similarly, Azmaiparashvili reported using latex solution injected through the major duodenal papilla for ductal dilation studies.
Biliary tree casts in humans and canines resemble a â??treeâ? formed by branches of varying calibers. Branching types include â??dendriticâ? (tree-like bifurcation) and â??magistralâ? (where smaller branches emerge sequentially from the main branch). In the radiographic images presented in our article, 65.9% of samples showed a magistral pattern, while 34.1% showed a dendritic pattern. This finding corroborates with Azmaiparashvili, who further described two subtypes within dendritic branching: Dichotomy and trichotomy, with dichotomy more frequently observed [9].
The present study aimed to determine an average size for the cystic duct length in dogs to help reduce surgical errors caused by the lack of anatomical knowledge of these structures. Regression analysis demonstrated that weight directly influences cystic duct length, as evidenced by a linear graph with r2 = 92.32%. We also observed that the type of gallbladder did not show a significant difference, with only a small variation in the angular coefficient (m = 0.01696). These findings strongly contrast with previous literature, where regression analysis suggested no significant correlation between cystic duct length and body weight [8]. Furthermore, we can estimate the cystic duct length in a given animal using only its weight, applying the following equation: Length = 0.0915 Ã? Weight.
Conclusion
Data analysis revealed that weight versus D-cis length shows a linear relationship with r2 = 92.32%, allowing for an estimated cystic duct length based on the patientâ??s weight. This approach may reduce the risk of postoperative bile leakage into the abdominal cavity during cholecystectomies. This study thus proposes a method to estimate the cystic duct length in dogs using only their weight.
We acknowledge the contributions of all authors, who participated in data collection, analysis, and manuscript preparation.
The authors declare no conflicts of interest.
We are grateful to CNPq for the recourses.