ISSN: 2347-7830
ISSN: 2347-7830
1Department of Life Sciences, National Cheng Kung University, Tainan, Taiwan
2Laboratory of Theoretical Ecology, Department of Entomology, National Chung Hsing University, Taichung, Taiwan
3Department of Environmental Engineering, Kung-Shan University of Technology, Tainan, Taiwan
4Both authors contributed equally
Received date: 12/01/2016; Accepted date: 14/03/2016; Published date: 18/03/2016
Visit for more related articles at Research & Reviews: Journal of Ecology and Environmental Sciences
Both temperature and salinity significantly affected the demographic traits of the snail Thiara riqueti (Grateloup) (Mesogastropoda, Thiaridae). At 5% salinity, snails survived less than a week and no reproduction occurred. At 10ºC, the mean longevity of the snail was about 10 days and no reproduction was observed. At 18ºC, total longevity ranged from 4.1 to 19.8 weeks, and only minor reproduction was observed at 1% salinity. The longevity at 10 and 18ºC, however, is sufficient for the snail to survive the short, mild winter period in Taiwan. At 25ºC and 30ºC, reproduction was observed at all salinities (except at 25ºC and 0.2% salinity). At 30ºC, the intrinsic rates of increase were 0.1236, 0.1502, 0.1081 and 0.094 for salinity 1.0%, 2.0%, 2.5% and 3.0%, respectively. The net reproductive rate at 30ºC ranged from 0.92 to 56.3 offspring per individual. Although T. riqueti was reported as a freshwater snail, our results indicate that it is euryhaline in Taiwan.
Demography, Life history, Intrinsic rate of increase, Thiara riqueti.
Snails play important roles in aquatic ecosystems. Hall [1] reported that the exotic freshwater snail (Potamopyrgus antipodarum) consumed 75% of gross primary productivity, and their excretion accounted for two-thirds of ammonium demand in a highly reproductive stream in Wyoming, US. Gutiérrez [2] pointed out that shells of snails are substrata for attachment of epibionts, provide refuges from predation, and control transport of solutes and particles in the benthic environment, and thus changes in shell production have important consequences for other organisms.
Thiara (Sermyla) riqueti (Grateloup, 1840) (Mesogastropoda, Thiaridae) is distributed from the western coast of India, southeast Asia, Malaysia, Australia, the Philippines, China, Taiwan and the Ryukyu Islands [3]. Although Pace [3] reported it as an inhabitant of freshwaters, Cheng [4] found it to be the most abundant species in the saline environment of Su-Tsao estuary, Tainan, Taiwan. It is one of the major prey species of several birds, including the black-winged stilt (Himantopus himantopus [Linnaeus, 1758]) and the pied avocet (Recurvirostra avosetta [Linnaeus, 1758]) (Ueng, personal communication).
The population dynamics of aquatic organisms is under the influence of diverse environmental factors; among them, water salinity and temperature are generally considered as the dominant “ecological master factors” [5-8]. Because the survival and fecundity of T. riqueti vary with the age, its population dynamics cannot be properly described or analyzed by using the exponential model or logistic model. For an age-structured population, the cohort life table gives a detailed and complete description of the survivorship, development, and reproduction of a population. For a comprehensive understanding of the effect of temperature and salinity on population dynamics of T. riqueti, life table study is of prime importance. However, due to the difficulty, tediousness and time-consuming work in collecting the age-specific survival rate and fecundity in a life table study, only a few data were available for snails.
The traditional life history theories [9-12] deal only with the female age-specific survival rate and fecundity and ignore the male population and the variable developmental rate that occurs among individuals. Chi and Liu [13] and Chi [14] demonstrated that agespecific life tables could not properly describe the stage differentiation of a population. They developed an age-stage, two-sex life table to present an age-stage description for the complete life history and to incorporate variable developmental rates occurring among individuals. Yu et al. [15] and Chi and Su [16] gave mathematical proofs on the relationship between female fecundity, preadult survival rate, and the gross reproductive rate.
In Taiwan, aquaculture of oyster, fishes, and eels significantly affected the costal and estuary habitats. For an integrated conservation and habitat restoration, we need urgently the ecological information for most costal organisms. Among many ecological studies, life table are the most basic and important, though very difficult, subject. For a preliminary understanding of the population ecology, we studied the field dynamics for two years. In accompany with the field study, we studied the cohort life table of T. riqueti at different temperatures and salinities in the laboratory and analyzed the raw data by using the age-stage, two-sex life table.
Thiara riqueti (Grateloup) were hand collected from the Su-Tsao estuary. The snails were transferred to 9-cm diameter Petri dishes containing sea water and kept in growth chambers at different temperatures (10 ± 1°C, 18 ± 1°C, 25 ± 1°C and 30 ± 1°C) for 2 d. A total of 28 treatment conditions, i.e., seven salinities (0.2%, 1.0%, 2.0%, 2.5%, 3.0%, 4.0%, 5.0%) and four temperatures (10°C, 18°C, 25°C, 30°C), were used in this study. Forty-eight newborn snails laid within a two day period were used for the life table study at 18°C and 25°C, 36 newborns for 10°C, 48 to 72 newborns for 30°C (Table 1). Because snails did not reproduce at 10°C, newborns collected from 18°C were used for the 10°C life table study. Each newborn snail was kept in an individual well of a 24-well cell culture cluster (cell diameter 1.5-cm) (Becton Dickinson Labware) filled with varying rearing solutions. The rearing solutions of different salinities were prepared from seawater and reverse osmosis water. The salinity of seawater was measured by using a conductivity meter (Model LF 330, WTW Wissenschaftlich-Technische Werkstatten, Weilheim, Germany). Commercial sea salt was used for preparating rearing solution of higher salinities. Rearing solutions were replaced weekly. Three species of algae, including the two predominant species Ulothrix flacca (Dillwn) Thuret in Le Jolis, Enteromorpha intestinalis (Linnaeus) Nees, and a minor species, Cladophora albida (Huds.) Kutz, grew on the interval surface of the well and served as food for T. riqueti. Because of the increase in size, after 3 mo the snails were moved to the larger wells of a 6-well cell culture cluster (cell diameter 3.5-cm) containing rearing solution. The mobility and fecundity were recorded weekly until the death of all individuals, except for the 10°C life table study, where mobility and fecundity were recorded daily. The death of a snail was judged according to the isolation of operculum and the decomposition of the body. If a snail was judged as dead, then the date of death was counted backwards to either the first week of the consecutively immobile period or the last reproduction week. The life history is divided into two stages; the pre-reproductive stage (the period prior to the first reproduction), and the adult stage (the period following the initiation of reproduction). Snail lengths were measured monthly. All experiments were carried out in growth chambers set at the above temperatures and a L:D=12:12 h photoperiod.
| Temperature/ Salinity | n01 | Adult stage (d = day, w = week) | Total longevity (d = day, w = week) | Fecundity3 (offspring) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SE2 | n | Mean | SE | n | Mean | SE | ||
| 10ºC/0.2% | 36 | 0 | - | - | 36 | 10.1 d | 0.5 | 0 | - | - |
| 10ºC/1.0% | 36 | 0 | - | - | 36 | 11.0 d | 0.3 | 0 | - | - |
| 10ºC/2.0% | 36 | 0 | - | - | 36 | 10.9 d | 0.2 | 0 | - | - |
| 10ºC/2.5% | 36 | 0 | - | - | 36 | 10.8 d | 0.2 | 0 | - | - |
| 10ºC/3.0% | 36 | 0 | - | - | 36 | 11.0 d | 0.3 | 0 | - | - |
| 10ºC/4.0% | 36 | 0 | - | - | 36 | 10.8 d | 0.3 | 0 | - | - |
| 18ºC/0.2% | 48 | 0 | - | - | 48 | 4.9 w | 0.4 | 0 | - | - |
| 18ºC/1.0% | 48 | 1 | 44 w | - | 48 | 19.8 w | 3.3 | 1 | 4 | - |
| 18ºC/2.0% | 48 | 0 | - | - | 48 | 14.2 w | 2.3 | 0 | - | - |
| 18ºC/2.5% | 48 | 0 | - | - | 48 | 9.3 w | 0.5 | 0 | - | - |
| 18ºC/3.0% | 48 | 0 | - | - | 48 | 9.1 w | 0.7 | 0 | - | - |
| 18ºC/4.0% | 48 | 0 | - | - | 48 | 4.1 w | 0.3 | 0 | - | - |
| 25ºC/0.2% | 48 | 0 | - | - | 48 | 10.0 w | 1.1 | 0 | - | - |
| 25ºC/1.0% | 48 | 1 | 58 w | - | 48 | 14.0 w | 2.3 | 1 | 167 | - |
| 25ºC/2.0% | 48 | 10 | 30.4 w | 4.9 | 48 | 22.3 w | 3.0 | 10 | 9.9 | 2.7 |
| 25ºC/2.5% | 48 | 5 | 25.0 w | 4.5 | 48 | 16.3 w | 2.2 | 5 | 9.8 | 3.1 |
| 25ºC/3.0% | 48 | 7 | 13.0 w | 2.0 | 48 | 16.8 w | 1.2 | 7 | 1.6 | 0.2 |
| 25ºC/4.0% | 48 | 3 | 10.3 w | 2.6 | 48 | 14.0 w | 1.8 | 3 | 1.7 | 0.7 |
| 30ºC/0.2% | 48 | 9 | 44.6 w | 10.6 | 48 | 18.6 w | 3.9 | 9 | 16.2 | 4.3 |
| 30ºC/1.0% | 48 | 29 | 36.1 w | 6.3 | 48 | 37.8 w | 5.2 | 29 | 49.6 | 11.9 |
| 30ºC/2.0% | 48 | 47 | 40.2 w | 4.7 | 48 | 39.0 w | 4.1 | 47 | 79.1 | 13.8 |
| 30ºC/2.5% | 72 | 31 | 20.6 w | 5.7 | 72 | 15.8 w | 2.8 | 31 | 29.0 | 10.8 |
| 30ºC/3.0% | 71 | 25 | 19.4 w | 4.3 | 71 | 15.1 w | 2.0 | 25 | 23.6 | 8.2 |
| 30ºC/4.0% | 48 | 7 | 8.1 w | 1.9 | 48 | 13.3 w | 0.9 | 7 | 6.3 | 1.4 |
1: n0: total number of individuals used at the beginning of life table study.
2: Standard error cannot be calculated when only one individual survived this stage.
3: Fecundity is calculated by using those individuals that developed to the adult stage.
Table 1: Duration of the adult stage (the reproductive stage), the total longevity (d: day, w: week) and the fecundity (offspring) of T. riqueti at different temperatures and salinities.
The raw life history data for each individual, i.e., the length of the pre-reproductive stage and the adult stage, and the weekly fecundity of the adult stage, were pooled and analyzed according to the method described in Chi and Liu [13] and Chi [14]. In order to avoid the tedious efforts involved in raw data analysis, a computer program TWOSEX-MSChart (Chi, 2015) designed in Visual BASIC version 6 for the Windows operating system is available at http://140.120.197.173/Ecology/ and http://nhsbig.inhs.uiuc. edu/wes/chi.html (Illinois Natural History Survey). TWOSEX-MSChart analyzes the raw data and calculates all life table statistics; e.g, the age-stage specific survival rate (sxj; where x = age and j = stage), the age-stage specific fecundity (fxj), the age-specific survival rate (lx), the age-specific fecundity (mx). The population parameters (r, the intrinsic rate of increase; λ, the finite rate of increase, λ= er; R0, the net reproductive rate; T, the mean generation time) are calculated accordingly. The net reproductive rate is calculated as: 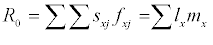 Intrinsic rate of increase was estimated by using the iterative bisection method from the Euler-Lotka formula:
Intrinsic rate of increase was estimated by using the iterative bisection method from the Euler-Lotka formula: 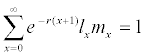 with age indexed from 0 (Goodman, 1982). The life expectancy (exj) was calculated according to Chi and Su [16]. The mean generation time (T) is defined as the length of time that a population needs to increase to R0-fold of its size (i.e., erT = R0 or λT = R0) as the stable increase rate (the intrinsic rate r and the finite increase rate λ) is reached. The mean generation time is calculated as T = lnR0/r. The variances and standard errors of the population parameters were estimated by using the bootstrap procedure [18,19] during the life table analysis by using the TWOSEX-MSChart program [20].
with age indexed from 0 (Goodman, 1982). The life expectancy (exj) was calculated according to Chi and Su [16]. The mean generation time (T) is defined as the length of time that a population needs to increase to R0-fold of its size (i.e., erT = R0 or λT = R0) as the stable increase rate (the intrinsic rate r and the finite increase rate λ) is reached. The mean generation time is calculated as T = lnR0/r. The variances and standard errors of the population parameters were estimated by using the bootstrap procedure [18,19] during the life table analysis by using the TWOSEX-MSChart program [20].
The means and standard errors of the duration of the adult stage, the total longevity and fecundity are given in Table 1. Because T. riqueti survived less than one week and did not reproduce at 5% salinity, the data for 5% salinity are not listed in Table 1and are excluded from the following discussion. At 10°C, the total longevity of T. riqueti was only about 10 days and it failed to reproduce at all salinities. At 18°C, the total longevity ranged from 4.1 to 19.8 weeks, and only a few offspring (4) were produced at only one salinity (1%). At 25°C, T. riqueti reproduced at most salinities except 0.2% salinity (Table 1). An extraordinarily high fecundity (167 offspring) and long adult duration (58 wk) were recorded for the single female that emerged at 25°C and 1.0% salinity. At 30°C, T. riqueti reproduced successfully at all salinities. The maximum mean fecundity (79.1 offspring) and the longest mean longevity (39.0 wk) were obtained at 30°C and 2.0% salinity.
The age-stage-specific survival rate (sxj) for the pre-reproductive stage (P) and the female adult (A), and the age-specific fecundity (mx) of adult T. riqueti at 25°C and 30°C are shown in Figures 2 to 7 for different salinities. The age-stage-specific survival rate (sxj) gives the probability that a newborn will survive to age x and stage j. At 25°C, the first reproduction began within 20 wk for salinities 2.0%, 2.5% and 3.0%. However, at low salinity (1.0%) it was recorded after 50 wk (Figure 3) and at high salinity (4.0%) after about 26 wk (Figure 7). At 30°C, reproduction began about 10 weeks after birth and lasted as long as 60 to 120 wk for salinity ranging from 0.2% to 3.0%.
Population parameters at different temperatures and salinities are listed in Table 2. Because T. riqueti did not produce offspring at 10°C and 18°C (except 18°C and 1% salinity), no intrinsic rate can be estimated for these temperatures. At 18°C and 1% salinity, the net reproductive rate (R0) is 0.083 offspring; the intrinsic rate of increase is -0.0218 wk-1. At 25°C and 30°C, T. riqueti has a positive intrinsic rate of increase for all salinities, except 25°C/3.0% salinity and 25°C/4% salinity. In comparison to 25°C, life tables at 30°C were characterized by faster individual development, higher intrinsic rate of increase (r) and higher net reproductive rate (R0). Higher intrinsic rates are found at 30°C with the value 0.1236, 0.1502, 0.1081 and 0.0946 for salinity 1.0%, 2.0%, 2.5% and 3.0%, respectively. The intrinsic rates of increase at 30°C fit a quadratic equation (R2 = 0.9552) (Figure 8).
| Temperature/ Salinity | Intrinsic rate of increase (r) (week-1) (jackknife) | Intrinsic rate of increase (r) (week-1) (bootstrap) | Net reproductive rate (R0) (offspring) | Mean generation time (T) (week) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SE* | Mean | SE* | Mean | SE* | Mean | SE* | ||
| 18ºC/1.0% | -0.0218 | - | 4 | - | 0.08 | - | 114.2 | - | |
| 25ºC/1.0% | 0.0164 | - | 167 | - | 3.48 | - | 76.1 | - | |
| 25ºC/2.0% | 0.0176 | 0.0090 | 10.6 | 3.0 | 2.06 | 0.79 | 46.1 | 2.5 | |
| 25ºC/2.5% | 0.0046 | 0.0127 | 15.6 | 3.0 | 1.04 | 0.54 | 51.8 | 4.7 | |
| 25ºC/3.0% | -0.0656 | 0.0184 | 0.89 | 0.29 | 0.23 | 0.09 | 21.2 | 2.5 | |
| 25ºC/4.0% | -0.0572 | 0.0299 | 0.66 | 0.41 | 0.10 | 0.07 | 33.2 | 1.5 | |
| 30ºC/0.2% | 0.0275 | 0.0102 | 26.5 | 4.90 | 3.04 | 1.20 | 43.6 | 2.8 | |
| 30ºC/1.0% | 0.1244 | 0.0107 | 175.7 | 45.7 | 29.9 | 8.0 | 27.5 | 2.9 | |
| 30ºC/2.0% | 0.1504 | 0.0074 | 294.7 | 44.8 | 56.3 | 10.8 | 27.0 | 1.4 | |
| 30ºC/2.5% | 0.1091 | 0.0164 | 175.8 | 59.6 | 12.5 | 4.9 | 23.8 | 3.9 | |
| 30ºC/3.0% | 0.0963 | 0.0178 | 94.2 | 34.0 | 8.42 | 3.19 | 22.9 | 2.8 | |
| 30ºC/4.0% | 0.0016 | 0.0412 | 1.64 | 0.69 | 0.92 | 0.38 | 11.3 | 0.5 | |
Table 2: Population parameters of T. riqueti at temperature (25°C and 30°C) and salinity (0.2, 1.0, 2.0, 3.0, 4.0ïùê) based on age-stage, two sex life table in the laboratory
At 10°C and 18°C, the total longevity of T. riqueti was short and they did not produce offspring (with a minor exception occurring at 18°C /1% salinity), explaining the low density found for this species in winter. However, the longevity at 10°C and 18°C was adequate for it to survive the short, mild winter period in Taiwan. At 25°C and 30°C, T. riqueti survived and produced offspring at most salinity (Table 1). These results indicate that T. riqueti is euryhaline, and explains why T. riqueti is found in estuarine areas. This is consistent with the literature report by Abbott [21] classifying T. riqueti as a brackish water species.
Population parameters calculated from life table studies reveal the life-long effect of a variety of factors on the population. They summarize the joint effect of age-specific survival rate and fecundity on population growth. However, the tedious calculation inherent in life table analysis may result in errors in population parameters, and, consequently, affect interpretation. Ferrando et al. [22] studied the effect of the toxicant 3,4-dichloroaniline (DCA) on the freshwater rotifer Brachionus calyciflorus and their results showed that R0 > 1 and r < 0 for the treatment of 10 ppm DCA (Figure 3 in Ferrando et al. [22]). According to life table theory, R0 > 1 must always be accompanied with r > 0. Thus, it was an obvious error in their results. Nandini and Sarma [23] studied the life table of four cladoceran species in relation to algal food density. In their report, the gross reproductive rate (GRR) for Ceriodaphnia cornuta is 25.8 eggs/female at food density of 0.5×106 cells/ml (Table 1 in Nandini and Sarma, [23]). Because the GRR is the simple summation of the age-specific fecundity (mx) over all ages, it can be easily estimated from the fecundity curve. According to the fecundity curve (Figure 1 in Nandini and Sarma, [22]), the GRR at food density of 0.5×106 cells/ml should be obviously much greater than the reported 25.8 eggs/female, indicating that their data may be in error. In our study, negative values of intrinsic rate were obtained at 18°C/2.0% salinity, 25°C/3.0% salinity, 25°C/4.0% salinity, and 30°C/4.0% salinity (Table 2). The respective net reproductive rates (R0) were all less than one. These results are consistent with the theoretical relationship, i.e., if R0 < 1 then r < 0. Because life table studies are extremely time-consuming, eg, the life table of T. riqueti at 30°C and 2.0% salinity took more than two years, the jackknife method and bootstrap technique have been used to estimate the mean and standard error of population parameters. Huang and Chi [24] proved, however, that the jackknife method should not be used for the estimation of population parameters. A few problems were also noticed in our application of the jackknife method in this study. At 30°C and 4.0% salinity, the estimated mean of intrinsic rate is 0.0016 d-1, and the mean of the net reproductive rate is 0.92 offspring. Because, according to life table theory that when R0 <1 r should always be < 0, the results obtained by using the jackknife method for these values are inconsistent with the life table theory. However, when the bootstrap method is used, it yields an intrinsic rate of -0.0077 d-1 and a net reproductive rate of 0.92. The results are then consistent with the life table theory.
Dudgeon [25] studied the population dynamics of the thiarid Melanoides tuberculata (Muller, 1774) and found there was a single peak in juvenile recruitment coinciding with the warmer months in Hong Kong. Dudgeon (1989) reported T. scabra is semelparous, parthenogenetic and viviparous. Haynes [27] reported that the thiarid Fijidoma maculata (Mousson) reproduced offspring parthenogenetically throughout the year in Fiji. Our results show that T. riqueti is iteroparous. Apparently there is abundant variation in reproduction strategy among different species of Thiaridae.
Life tables collected under different environmental conditions are useful for the explanation of field population dynamics. In most ecology textbooks, the intrinsic rate of increase (r) is used to describe the characteristics of a population as r-selected or K-selected. Dudgeon (1982) reported that there were two peaks of recruitment per annum for Brotia hainanensis (Brot, 1872). He pointed out that the preadult mortality is high due to severe seasonal spates, and the life cycle of this snail is typical of an r-selected species adapted to conditions of high density-independent mortality. Our results show that T. riqueti is an r-selected species.
Liang [29] built a structured equation model for physiochemical variables of water, benthic invertebrates, and feeding activity of waterbirds in Sitsao (now named Su-Tsao) wetlands in Taiwan. Because their model is based only on statistical analysis without quantitative equations, its practical application is limited. Incorporation of life tables of benthic invertebrates will certainly help in revealing their population dynamics and biomass production. Raut [30] simulated the population growth of Achatina fulica (Gastropoda: Achatinidae) based on a life table and found 100 individuals may potentially give rise to 1.26 × 1012 individuals through successive generation within 2700 days. Such long-term projection of population growth will give meaningful results only when the variability of the life table at different conditions is taken into consideration
Life tables give the most comprehensive description of the survival, development and reproduction of a population. Without life tables of different environments we cannot properly interpret population dynamics in the wild. However, life table traits vary with many different factors, and it is a very tedious and time-consuming process to collect life table data under all different environmental conditions. Maranhão and Marques [8] found both the duration of embryonic development and the number of juveniles produced per female of Echinogammarus marinus Leach (Gammaridae) were significantly affected by temperature. Marques [5] also observed that the fecundity of Cyathura carinata (Krøyer) was correlated with female size. Life tables are also important for the study of predator-prey relationships. Weider and Pijanowska [31] conducted life table experiments to study the plasticity of Daphnia life histories in response to chemical cues from predators. Walls and Ventelä [32] studied the life history variability in response to temperature and Chaoborus exposure in three Daphnia pulex clones and found that predator exposure and temperature significantly affected age and size at first reproduction and the total number of offspring in Daphnia. For use in the aquaculture of shrimp and fishes, Wang [33] studied the life table of the prey water flea Moina macrocopa Straus at different salinity and food conditions. Ahmad [34] studied the optimum feeding rate of the rotifer Brachionus plicatilis on the marine alga Nannochloropsis sp. using life tables. Garton and Stickle [35] showed that the predation rate of Thais haemastoma on oyster spat is sensitive to temperature and salinity. Chi and Yang [36] studied the stage-specific predation rate of the predator ladybird beetle Propylaea japonica Thunberg using the life table. These reports show that life tables can be used not only to evaluate the potential of a population in response to different environmental factors, but also to study the predator-prey relationship for aquaculture and other applications. These reports also show, however, that the effects of many factors which play important roles in the life tables of many organisms remain unknown. This is true in T. riqueti as well as most other organisms.
Cheung and Lam [37] showed that temperature, salinity and their interactions were significant in affecting the respiration rate of Nassarius festivus (Powys, 1835) (Gastropoda: Nassariidae). Berry and Hunt [38] showed that adults and juveniles of Littorina rudis (Maton) showed different tolerance to salinity and temperature. The yearly water temperature at Su-Tsao estuary ranged from about 15 to 35 °C and the salinity from 0.5 to 4.5%. Our results demonstrate that both temperature and salinity have significant effects on the population dynamics of T. riqueti. However, in our study T. riqueti was kept at constant salinity and temperature. The effect of fluctuating salinity and temperature on T. riqueti deserves further study. Nevertheless, our results possibly explain why the snail population almost disappears during the winter in Su-Tsao estuary when the temperature is low and before the rainy period when the salinity is high. They also explain the high fecundity, pregnancy and population density of T. riqueti in summer and fall as well. The optimal temperature and salinity combination observed in the life table study in the laboratory confirms the increase of the snail population during the warm and rainy period in Su-Tsao estuary [4]. The intrinsic rates of increase at 30°C showed that both low and high salinities have an adverse effect on the T. riqueti population.
For predicting fluctuations in snail population, not only the life table but also the dispersal, the predation, the competition and other factors are required. For example, Pointier [39] studied the invasion dynamics of the Oriental thiarid snail Thiara granifera in the Martinique island, French Antilles and found colonization was faster downstream than upstream. Dudgeon [25] studied the population dynamics and productivity of the thiarid Melanoides tuberculata (Muller, 1774) in Hong Kong and indicated that parthenogenicity may enhance the retention of favorable adaptations and ensure a rapid spread through an expanding population. Myers [40] reported that thiarids are parthenogenetic and viviparous, and immature snails released from the adult stay in close proximity to their mothers and rely on passive dispersal for colonization of new habitats. Sexual reproduction, however, has been reported for the thiarid Melanoides tuberculata in Israel [41]. Chaniotis et al. [42] reported the presence of males in Puerto Rican Thiara (Tarebia) granifera which was previously thought to be only parthenogenetic. In our study, we did not dissect the snails which did not reproduce to check their sexes. However, because we used the two-sex life table and all individuals were included for the analysis, the uncertainty of parthenogenesis will not affect the analytical results of population parameters. Nevertheless, both the biology and ecology of many thiarids deserve further study. In Hong Kong, Dudgeon [43] reported that mollusks dominated the reservoir benthos and the most encountered gastropods were Thiara scabra, Melanoides tuberculata, Sinotaia quadrata and Radix plicatulus. Lai [44] reported the succession of algae and gastropods in autumn of Su-Tsao wetlands and concluded the seasonal variation of dominant benthic algae was controlled not only by water salinity but also by the population of herbivorous gastropods. Macro-invertebrates may play an important role in energy transfer and constitute an important prey for upper trophic levels [39,45]. For successful conservation practices in the future, we strongly recommend the necessity of studying the population dynamics of component species at different trophic levels of a food chain based on the life tables of their member species.
We thank Dr. Sheue-Duan Lai for identification of algae. We thank Dr. Cecil L. Smith for correcting English. This research is supported by a grant from the Forestry Bureau, Council of Agriculture, Taiwan to Wang JP.