e-ISSN: 2347-7857 p-ISSN: 2347-7849
e-ISSN: 2347-7857 p-ISSN: 2347-7849
Bapatla College of Pharmacy, Bapatla, Guntur District, Andhra Pradesh, India
Received date: 25 November 2013 Accepted date: 24 December 2013
Visit for more related articles at Research & Reviews: Journal of Pharmaceutics and Nanotechnology
Microencapsulation by ionic gelation technique is an approach to achieve controlled release of drug, gliclazide beads were designed to improve the absorption and bioavailability of the drug. Gliclazide beads were formulated with sodium alginate and by combination of sodium alginate with hydrophilic polymer such as Na CMC in the ratio of (core:coat) 1:1,1:2, 1:3, 2:1 and 3:1 by ionic gelation method.The prepared beads were characterized such as particle size, angle of repose, compressibility index, hausner’s ratios, wall thickness, drug content,entrapment efficiency. The shape of beads were found to be spherical by SEM analysis. In-vitro dissolution data revealed that formulations exhibited the zero order kinetics and followed peppas transport mechanism.
Gliclazide, Na CMC- Sodium Carboxy Methyl Cellulose,Ionic gelation method,SEM-scanning electron microscopy
In recent years considerable attention has been focused on the development of new drug delivery systems known as controlled release drug delivery systems. Such interest is based largely on the fact that the controlled release products have established and retained a place in the market based on their uniqueness and their clinical advantages in the practices of medicine.
Controlled release drug delivery systems [1,2] are those dosage formulations designed to release an active ingredient at rates, which differ significantly from their corresponding conventional dosage forms. The controlled release drug delivery systems are aimed at controlling the rate of drug delivery, sustaining the duration of therapeutic activity and/or targeting the delivery of the drug to a tissue. Drug release from these systems should be at a desired rate, predictable and reproducible. Microencapsulation is a process whereby small, discrete solid particles or liquid droplets are surrounded and enclosed by an intact shell to result in microcapsules. The concept of microencapsulation was initially utilized [3,4,5] in carbonless copy papers.
Gliclazide [6,7] is an oral hypo glycemic second generation sulfonyl urea drug which Is useful for a long term treatment of non insulin dependent diabetes mellitus (NIDDM). Previous studies showed that gliclazide possesses good general tolerability, low incidence of hypoglycemia and low rate of secondary failure. Rapid absorption from the GIT is required for the oral hypoglycemic drug for effective therapy. However, the absorption rate of Gliclazide from the GIT is slow and varied among the subjects. Slow absorption has been suggested to be due to either poor dissolution of Gliclazide due to hydrophobic nature of drug and poor permeability of drug across the GI membrane [8,9]. By incorporation of Gliclazide in the alginate beeds may control its absorption from GI tract and overcome the variability problems. Thus this study was undertaken to develop controlled formulations of Gliclazide using sodium alginate and Na CMC as release retardant polymers.
Gliclazide was received from hetro drugs, Hyderabad as gift sample, sodium alginate was procured from S.D fine chem., Na CMC from yarrow chem. Products and all other chemicals and solvents used in this study were LR grade.
Preparation of gliclazide alginate beads
Gliclazide beads were prepared by ionic gelation method [10,11] with sodium alginate alone and combination of sodium alginate with Na. CMC in the core: coat ratio of 1:1, 1:2, 1:3, 2:1 and 3:1as shown in table no 1 and 2. Sodium alginate powder is mixed with 30 ml of purified water and allowed to stirring for 30 mins. The required amount of gliclazide was thoroughly mixed with sodium alginate at 400 rpm in a beaker. The resulting dispersion was added drop wise into beaker containing 100ml of 10%w/v calcium chloride solution through a syringe with a needle size no.18 and the solution was stirred at 400 rpm. A Remi medium duty stirrer was used for stirring and the stirring was continued for 30 mins to complete the curing reaction and the product was separated, washed with water. The collected beads were dried at 450C for 12 hrs. Similarly glicazide alginate beads were also prepared by the combination of sodium alginate with hydrophilic polymer such as Na CMC in the core: coat ratio of 1:1, 1:2, 1:3, 2:1, and 3:1 and subjected to evaluation studies.
Infrared spectroscopy
Compatibility studies between gliclazide and polymers were studied by FTIR spectroscopy with KBr pellet method and the spectrums were recorded for the samples in the wavelength region of 4000 to 400 cm−1.
Evaluation of Alginate Beads
The following evaluation studies are conducted on prepared gliclazide beads.
Size Analysis [12]
Particle size distribution and the mean diameter of the beads were determined by sieving method. The beads were sieved by taking the standard IP set of sieves of 10, 12, 16, 22, 44, 60, and 100. The average particle size of the beads was calculated by using the following equation.
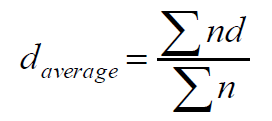
Where n = frequency weight
d = mean size.
Evaluation of Flow Properties
The flow properties of different beads were studied by measuring the angle of repose employing open tube method (2.3 cm diameter). The angle of repose was calculated by using the following formula
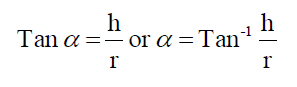
Where h = height of the pile, cm
r = radius of the base of the pile, cm
Bulk Density
Bulk density is the ratio of the beads to the bulk volume it occupies, expressed in gm/ml. 5 gm of the beads were weighed and poured into a 100ml- measuring cylinder and the volume was measured, it can be measurd by the following equation.

Tapped Density
Tapped density of beads determined by weighed accurately 5 gm of the beads were weighed and poured into a 100ml-measuring cylinder and the volume was measured. It was tapped mechanically for 100 times till a constant volume bulk volume obtained, which includes the true volume of beads and void space among the beads.
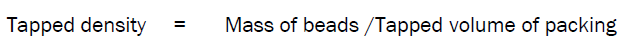
Carr’s Index [13]
The percentage of compressibility of beads was determined by Carr’s compressibility index.
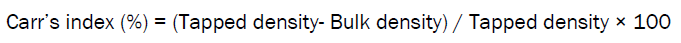
Hausner’s Ratio
Hausner ratio of beads determined by comparing the tapped density to the bulk density by using the equation

Wall thickness
Wall thickness of beads were determined by the method of Luu et al using the equation
Where h is the wall thickness
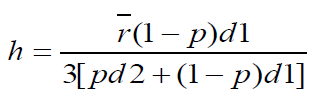
r = Arithmetic mean radius of the microcapsules
d1 = Density of the core material
d2 = Density of the coat material
p = Proportion of the medicament in the microcapsules.
Drug Content
Gliclazide content in the alginate beads was estimated by an UV Spectrophotometric method based on the measurement of absorbance at 226 nm in phosphate buffer of pH 7.4. The method was validated for linearity, accuracy and precision.
Entrapment Efficiency
Entrapment efficiency was calculated using the formula.
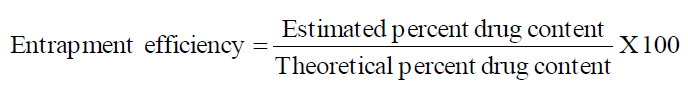
In- Vitro Drug Dissolution Study
The in vitro dissolution study was carried out by using USP Type 1 Dissolution apparatus containing 900 ml of phosphate buffer pH 7.4 as dissolution medium and maintained the temperature of the medium at 37±0.50c. The pre-weighed beads were then introduced into the dissolution basket and rotated at 100 rpm. At different time intervals, 5 ml of aliquates were withdrawn and filtered through 0.45 μm milli pore filter unit and analyzed spectrophotometrically at 226 nm and at each time of withdrawal, 5 ml of fresh dissolution medium was replaced into the dissolution flask to maintain the sink conditions.
Mucoadhesion Testing By In- Vitro Wash-off Test [14]
The Mucoadhesive property of the alginate beads was evaluated by an in-vitro adhesion testing method known as wash-off method. In this method Pieces of intestinal mucosa (2x2 cm) were mounted on to glass slides (3x1 inch) with cyanoacrylate glue. Two glass slides were connected with a suitable support. About 50 beads were spread on to each wet rinsed tissue specimen and immediately thereafter the support was hung on to the arm of a USP tablet disintegrating test machine, By operating the disintegrating test machine the tissue specimen was given a slow regular up and down moment in a test fluids at 37 OC taken in a one lit vessel of the machine. At the end of every 1 h the machine was stopped and the number of microcapsules still adhering on to the tissue was counted for 8h the test was performed at both gastric pH 1.2 and intestinal pH7.4.
SEM Analysis
SEM was performed for morphological characterization of microcapsules using scanning electron microscope (SEM—LEICA, 5430, London, U.K). They were mounted directly on to the SEM sample stub using double - sided sticking tape and coated with gold .lm (thickness, 200 nm) under reduced pressure (0.001 mmHg).
The drug-excepient compatibility studies were conducted by FT-IR spectroscopy, results revealed that no chemical interactions were observed between the drug and polymers as given in fig, 4, 5, 6 and 7.
The results of physical characteristics of formulated gliclazide beads were shown in the table no 3 and 6. The particle size of the gliclazide were found in the range of 1086.92 to 1497.52 μm. Results of the angle of repose for the formulations indicates that formulations exhibited good flow properties, further supported by carr’s index and hausner’s ratio. From the observation the particle size increased while increasing the concentration of polymer coat and flow properties. Topographical studies were conducted by SEM analysis as given in fig 8 ,which indicates surface of the coat have smooth surface and completely covers with a coat material, found to be spherical in shape.The wall thickness of gliclazide beads found to be increased while increasing the coat thickness that is concentration of polymer that exists good correlation ship between wall thickness and release rate constant as shown figure 2. All formulations have maximum percentage of drug content and good entrapment efficiency due to ionic gelation process. The formulations were also subjected to In-vitro wash off test in presence of 0.1N HCl and pH 7.4 phosphate buffers. The wash off was relatively rapid in phosphate buffer pH 7.4 than in acid buffer pH 1.2. From the results of in-vitro wash off test indicated that beads have fairly good mucoadhesive property prepared by ionic gelation process,the results were shown in Table no 5 and 8. Gliclazide release from the beads were studied (In-vitro dissolution studies) in phosphate buffer of pH 7.4, release profiles were shown in fig 1 and 3. The in vitro release data fitted into zero order, first order , matrix and peppas equations. Drug release from the formulations IG3 and IG8 controlled the release for the period of 10 and 11 hrs among all the prepared formulations .The rate of drug release form the formulations followed zero order kinetics exhibited peppas transport mechanism, the obtained results given in table no 4 and 7 . The exponential coefficient values were found > 0.85 indicating the non fickian diffusion mechanism ,supercase II type release. Gliclazide alginate beads formulated with Na CMC have good mucoadhesive property than the formulations formulated with the sodium alginate alone, It was confirmed by the in vitro wash off test.
Gliclazide beads formulated by ionic gelation method employing sodium alginate and alone and sodium alginate with sodium CMC. In-vitro wash off test results indicates the beads formulated with Na CMC have good mucoadhesive property, which inturn helps to control the drug release for longer period of time. Drug release from the formulations followed zero order kinetics and exhibited peppas transport mechanism, hence the bioavailability of the drug were enhanced by this technique, this effects results in maintaining tight blood glucose levels and improved patient compliance.