e-ISSN: 2347-7857 p-ISSN: 2347-7849
e-ISSN: 2347-7857 p-ISSN: 2347-7849
Rohit Vijay Bhalke1, Kabir G Ghorphade2*
Department of Formulation Research and Development, Aurigene Pharmaceutical Services Limited, Hyderabad, India
Received: 22-Mar-2022, Manuscript No. JPN-22-55175; Editor assigned: 24- Mar-2022, Pre QC No. JPN-22-55175(PQ); Reviewed: 07- Apr-2022, QC No. JPN-22-55175; Accepted: 12-Apr-2022, Manuscript No. JPN-22-55175(A); Published: 19-Apr-2022, DOI:2347785710.4172/.10.1.001.
Visit for more related articles at Research & Reviews: Journal of Pharmaceutics and Nanotechnology
Co-processed excipients were prepared by precipitation method and melt granulation method. Co-processing is the way that new excipients coming to the market without undergoing rigorous safety testing of completely new chemical. It can be defined as combining two or more established excipients by an appropriate process. Co-processing of excipients could lead to formation of excipients with superior properties compared to simple physical mixtures of their components. The main aim of co-processing is to obtain product with an added value related to the ratio. Chitosan:Syloid XDP 3150 represents a potentially useful superdisintegrant in many pharmaceutical applications. Chitosan-Ozokerite wax represents a potentially useful floating time.The developed excipients were evaluated for compressibility index (Carr’s index), Hausner’ratio and Bulk density or Tapped density in comparison with physical mixture of excipients. The Bulk density and Tapped density was found to be (0.5 to 0.6) which indicate good flow in comparison to physical mixture of excipients due to granule formulation, Carr’s index in therange of the (49.2) and Hausner’ratio in the range of the (1.125).
In the present work we investigated co-processing Excipients were cop (C:S), chitosan and syloid XDP 3150, in weight ratios of 1:4 (w/w) and produced by direct mixing Differential Scanning Calorimetry (DSC), Fourier-Transform InfraRed (FT-IR), X-Ray Powder Diffraction (XRPD) and Scanning Electron Microscopy (SEM) techniques were used to characterize Cop-C:S and Cop- C:O , in addition to characterization of its powder and immediate release and controlled release dosage form.
Fast dissolving and Floating tablets of Metronidazole were prepared using the above co-processed excipients and evaluated for pre-compression and post–compression parameters. Among the tablets prepared, the co-processed Excipients Cop-(C:S), Cop-(C:O) at different ratio.
Co- processed excipients; Formation; Physical mixtures; Established excipients
Generally, excipients are added to pharmaceutical preparations for the purpose of improving powder physical properties, bulking up formulations and handling of incompressible active substances [1]. Therefore, excipients must accomplish good flow, cohesion or lubricating properties to powder blends that are necessary for the production of tablets by direct compression. The new types of excipients with improved functionality were developed by either physical modification and/or chemical modification of already existing materials. Amongst these types, co-processed excipients showed a remarkable industrial potential due to the multifunctionalities these excipients can provide with regard compressibility, compactibility and disintegration potential [2]. Co-processed excipient is a combination of two or more compendial or non-compendial excipients designed to physically modify their properties in a manner not achievable by simple physical mixing, and without significant chemical change. This leads to the formation of an excipient with superior properties compared to properties of the individual components [3]. In addition, these components undergo no alteration to their chemical structure upon co-processing as the final product is modified in a physical manner. Recently, new types of co-processed excipients based on co-processing of chitosan product on syloid XDP 3150 and Ozokerite wax have been investigated [4]. The findings reported major improvement to native syloid XDP 3150 and ozokerite wax, for e.g., to become an excipient with good flow, compactibility, and faster disintegration time, the properties of which native syloid XDP 3150 form thin chitosan:syloid XDP 3150 and were found to render co-processed excipients with super-disintegration potential. Chitosan:ozokerite wax was found to render co-processed excipients with floating time. Generally, a disintegrate and floating time is an agent, used in the preparation of tablets, which causes them to disintegrate are immediate release and their medicinal substances on contact with moisture. Superdisintegrants are more recently available and more useful than traditional disintegrants because they show superior disintegration action at lower concentration. Chitosan is considered one of the most valuable polymers for biomedical and pharmaceutical applications due to its biodegradability, biocompatibility, antimicrobial, non-toxicity, and anti-tumor properties. Nanoparticles, microspheres, hydrogels, films, and fibers are typical chitosan based forms for biomedical and pharmaceutical application. Examples of such application include nasal, ocular, oral, parenteral and transdermal drug delivery [5]. On the other hand, chitosan:syloid XDP 3150 have been tested as disintegration promoters, specifically the use of amorphous synthetic syloid XDP 3150. The high purity, high in adsorptive capacity- both for hydrophilic and hydrophobic compounds Syloid XDP 3150 is the responsible factor for its potential fast disintegrants criteria.
Showed that the combination of chitosan:syloid XDP 3150 with other superdisintegrants (crospovidone, sodium starch glycolate) lower the disintegration time without affecting the tablet hardness [6]. Syloid XDP 3150 excipients are micronized synthetic amorphous silica gels of high purity that are widely formulated into many pharmaceutical products. This does not disintegrate by it but assist in quick capillary transportation of water deep inside compacts, thus lowers the disintegration time.
Metronidazole was gifted by Hetero-Bio pharma, Chitosan, Crospovidone LR (Kollidone), Sodium Starch Glycolate, Lactose, Ozokerite Wax, Micro Crystalline Cellulose, Manitol was gifted by research-lab fine chem. Industries Mumbai, India Syloid XDP 3150 was gifted by Grance, Germany.
Methods
Precipitation method: Chitosan (200 mg) were dispersed in 10 mL of 2 M hot (80°C) HCl solution for 10 minutes. Syloid XDP 3150 (800 mg) were dispersed in 10 mL of 2M NaOH solution to which 10 mL of distilled water were added under stirring until magnetic stirrer of the syloid XDP 3150 suspension was accomplished. The chitosan suspension was added gradually to the silica suspension. Under vigorous stirring, this was preceded for 1 h at ambient temperature (25°C) after completion of addition. The pH of the mixture was kept not to exceed, through mixing, a pH range from 6.5 to 7.0 by adjustment with concentrated HCl. The product was washed with deionized water. Then, the product was filtered out using filter papers. The filtrate was clear, as chitosan and/or Syloid XDP 3150 particle cause turbidity when present. The product was dried in the oven at 90°C to complete dryness and finally passed over #18-mesh. The product was stored in a well-closed container for further testing the characterizations of the excipient [7].
Melt granulation method: Chitosan powder (700 mg) was heated at a temperature of 75°C for 15 min with stirring. Ozokerite wax (300 mg) was melted at a temperature of 75°C for 15 min. Chitosan was added geometrically to melted ozokerite wax while mixing and keeping the temperature at 75°C for 10 min. The particles were then sieved while hot. Then they were cooled down to room temperature and then re-sieved again. The prepared Chitosan/ozokerite wax mixtures 40 mesh [8].
Analytical UV-Visible spectrophotometric method development tablets
UV-VIS spectrophotometric method development for metronidazole determination of ג max: The standard solutions of Metronidazole in different solvent media were scanned between 200-400 nm in UV spectrophotometer. The maximum absorbance was observed at 340 nm in water. The working ג max was chosen as 340 nm. The spectrum of metronidazole is shown in the figure.
Preparation of standard calibration curve of Metronidazole
Standard stock solution: Accurately weighed 10 mg of metronidazole standard was transferred to a volumetric flask and add sufficient water to produce 100 ml. After preparation of standard 1 ml of this solution was taken and made up to 50 ml with water, which gives 20 mcg/ml concentration (stock solution). From this stock solution concentration of 2,4,6,8 and 10 mcg/ml in water were prepared. The absorbance of the diluted solution were measured at 340 nm and a standard plot was drawn using the date obtained [9].
Preliminary trials for the preparation of co-processing excipients at disintegration time
Chitosan-Syloid 244 FP, Chitosan-Syloid XDP 3150 and Chitosan-Aerosil 200 at a disintegration time was measured to determine disintegration of co-processing Excipients using DT 1000 (Lab India).
Disintegration time of chitosan:syloid XDP 3150 co-processing at different ratio: Chitosan:Syloid XDP 3150 at different ratio 1:1, 1:2, 1:3, 1:4 the best ratio of 1:4 and 1% Lactose, to formulate a total tablet weight of 500 mg. the tablets were compressed using the single press tableting machine [10] to determine disintegration of co-processing excipients using DT 1000 (LabIndia).
Disintegration testing of super disintegrates: Chitosan: Syloid XDP 3150 co-processing excipients at different ratio, Crospovidone (2%), Sodium Starch Glycolate (2%) at to formulate a total tablet weight of 500 mg. the tablets were compressed using the single press tableting machine, to determine disintegration of co-processing excipients using DT 1000 (LabIndia).
Preliminary trials for the preparation of co-processing excipients at floating time
Cop-Chitosan:ozokerite wax, Cop-Chitosan:Carnuba wax, Cop-Chitosan:Compritol 888 ato, Cop-Chitosan:Cremophor RH 40, of different ratio the floating time measured to determine in-vitro dissolution of co-processing excipients (in 900 ml water) using Disso 8000 (LabIndia), 8-station dissolution test apparatus with a paddle stirrer at 50 rpm. A temperature 37 ± 0.5°C was maintained throughout the study.
Floating time chitosan:ozokerite wax co-processingat different ratio: Chitosan:Ozokerite wax co-processing of different ratio 7:3, 6:4, 9:1 the best ratio of (7:3) the floating time was measured to determine in-vitro dissolution of co-processing excipients (In 900 ml water) using Disso 8000 (Lab India), 8-station dissolution test apparatus with a paddle stirrer at 50 rpm[11]. A temperature 37 ± 0.5°C was maintained throughout the study.
Fundamental powder properties
Bulk and tapped densities, compressibility index and the hausner ratio: Bulk and Tapped Densities of each polymers and its corresponding co-processing were determined. An accurately weighed amount 3 g of each polymers powder was gently poured into a 50 ml graduated cylinder. The bulk volume was recorded and the bulk density (p bulk) was calculated. The same powder in the graduated cylinder was used to measure tapped densities. Tapped density (p tap) was determined by tapping the cylinder in a tapping device (in put the name) at least (100) times up to a constant volume. Tapped volume was then measured and the tapped density was calculated.
Bulk Density=weight of dry soil/volume of dry soil
Tapped Density=Weight of Powder/ minimum volume occupied after tapping
The compressibility (carr’s index) was calculated from the bulk and tapped densities according to equation
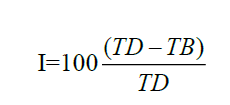
Carr’s Index=100 (Tapped Density- Bulk Density)/Tapped Density
On the other hand, Hausner Ratio (H) is an indirect of ease of powder flow. It is calculated by using the formula
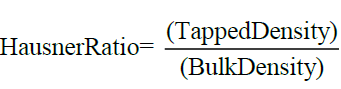
Characterization of the compression process
Kawakita analysis: Compressibility:Chitosan, Syloid XDP 3150, and Cop-(C:S) powder sample were compressed using a (Machine name---------) equipped with BB- punch and upper and lower punches as well as dies; Different compression force 500 to 800 (p) were applied .Three tablets were prepared to ensure reproducibility. Compression was carried out at 500 mg tablet weight. The compression behaviour of the sample was evaluated using Kawakita analysis [12].
Water penetration rate: Samples (1, 2, 3 and 5%) (wt/wt) were prepared by physically mixing Cop-C:S 1:4 with microcrystalline cellulose and manitol at a placebo mixture. The samples were poured individually into graduated cylinders to a fixed volume 10 ml without any applied pressure on the column of the resultant particles. The visualize and measure the water penetration into the samples 5 ml of sunset yellow solution was prepared and added to each of the prepared superdisintegrant samples. The penetration rate was calculated by measuring the speed of water (ml/sec.) penetrating the mixture columns. This test was further performed on crospovidone and sodium starch glycolate.
Testing the super disintegration power of chitosan-syloid-XDP 3150 among commercial super disintegrants: About 10 g of a standard placebo mixture was prepared by mixing Microcrystalline Cellulose and Manitol at a mass ratio of 7 g and 3 g respectively. Then 10 g of mixture made from the standard placebo and a tested superdisintegrat was prepared before compaction using the singal-press tableting machine at a pressure 500 p. The superdisinte grants tested were Chitosan-Syloid XDP 3150 and crospovidone, sodium starch glycolate all of which were added separately at different percentages (1%, 2%, 3%, 5%) to standard placebo mixture. Tablet disintegration was performed for each mixture and for the placebo powders itself compacted under the condition as the tested mixture.
Disintegration time was measured using a disintegration tester (DT1000 LABINDIA).
Swelling capacity: Swelling capacity was determined at room temperature for each polymer and its corresponding co-processing 3 g of each sample was transferred to a 25 ml graduated cylinder then the height of the powder bed was recorded (). Water (20 ml) was then added and the powder was allowed to swell (for about 24 h) before measuring the height of the swollen powder bed(hs) The swelling capacity was determined according to equation each determination was carried out in triplicate and result was expressed as mean ± SD.
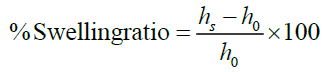
The experiment was repeated using physical mixture of each polymer (Syloid XDP 3150, Ozokerite was) with Chitosan at mass fraction contents similar to the ones used in the co-processing excipients.
Hydration capacity: Hydration capacity was determined at room temperature for each polymer and it’s corresponding, co-processing, according to the method of kornblum and stoppak.An accurately weighed amount of (0.5 g) of each sample was placed in 12 ml, centrifuge tube into which 8mL of water was added. The system was agitated for about 2 min then left to stand for 10 min before being centrifuged at 1,000 rpm. The weight of the sediment was recorded after decanting the supernatant. The percent hydration (W0) and weight after hydration (Wh) to the original material before hydration according to equation each determination was carried out in triplicate and result were expressed as mean ± SD.
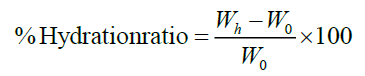
FTIR spectroscopy
Infrared spectroscopy of chitosan and Syloid XDP 3150, Cop-(C:S-1:4) , physical mixture of (C:S) at the mass ration of 1:1, and Cop-(C:O-7:3), physical mixture of Cop-(C:O) at the mass ratio of 1:1,Chitosan and Ozokerite wax can be operated in two modes A) ATR and B) transmittance mode.
ATR: For direct sampling of solids, semisolid and samples (only neutral pH).
Transmittance: For sampling of solids, semisolid and samples with KBr was recorded over a range 4000-400 cm-1 to study principal peaks using FTIR spectrophotometer (BRUKER) using model ALPHA T.
X-Ray diffraction
XRD study was performed to analyse crystalline or amorphous nature of the a) Cop-(C:S-1:4) b) chitosan and c) Syloid XDP 3150, A) Cop-(C:O-7:3) B) Chitosan and C) Ozokerite Wax were measured using X-ray diffractometer X-ray powder diffraction was recorded on BRUKER AXS D8 advance diffractometer system with a Cu Kα radiation (1.5406 Angstrom ) at 40 kV and 35 mA. Diffraction patterns were collected over 2θ range of 3-80° with a step size of 0.020° and step time 31:2 s.
SEM (Scanning Electron Microscopy)
The surface morphological properties of Chitosan, and Syloid XDP 3150, Cop-(C:S-1:4) samples were mounted. Investigated using a scanning electron microscope with Energy Dispersive Spectrophotometer (JSM-6360A, SEM-JEOL Instruments, Japan) used with Autofine CoaterJEOL JFC -1600.
UV-VIS spectrophotometric method development for metronidazole
Determination of ג max: The standard solutions of Metronidazole in different solvent media were scanned between 200-400 nm in UV spectrophotometer. The maximum absorbance was observed at 340 nm in water. The working ג max was chosen as 340 nm.
Preparation of standard calibration curve metronidazole: The calibration curve of Metronidazole in water (Figure 1) was found to be linear in the concentration range of 2-10 µg/ml having coefficient of regression (R2) of 0.9897 (Table 1).
| Sr.No. | Concentration (µg/ml) | Absorbance(nm) |
|---|---|---|
| 1 | 0 | 0 |
| 2 | 2 | 0.331 |
| 3 | 4 | 0.715 |
| 4 | 6 | 1.164 |
| 5 | 8 | 1.534 |
| 6 | 10 | 1.713 |
Table 1. Preparation of standard calibrationof Metronidazole.
Disintegration test result of preliminary trials
Co-processed excipients are various approaches have been employed to make the formulation challenges of poorly water-soluble drugs, such as precipitation method. Among these methods, porous silicate is a porous material that has been commonly used as a pharmaceutical excipient. Porous silicate having different characteristics such as particle size, pore size, disintegration and specific surface area are commercially available and have been widely employed to encapsulate poorly soluble drugs, such as Aerosil 200, Syloid XDP 3150, Syloid 244 FP. Various non-ordered mesoporous silica like Syloid XDP 3150 has been used to improve the disintegration properties of poorly soluble drugs. For this purpose to select of the different silica suspension Co-processed excipients (1:1) ratio are chitosan: Syloid XDP 3150, chitosan:Syloid 244 FP, chitosan:Aerosil 200 (Figure 2).
Disintegration time of co-processed chitosan:syloid XDP 3150 at different ratio
In order to select the optimal ratio and process for co-processed excipient preparation, of different ratios of chitosan and Syloid XDP 3150, four different ratios of Chitosan:Syloid XDP 3150 (1:1, 1:2, 1:3, 1:4). Direct mixing techniques were used. The prepared excipients were lubricated with lactose and compressed at tablet crushing forces (500 p). The tablets obtained were tested disintegration times versus Co-processing excipients (Figure 3).
The preliminary results of the aforementioned experiments indicated that direct mixing were suitable, the mixtures prepared by direct mixing displayed Fast disintegration properties.
Disintegration testing of superdisintegrant
The use of co-processing is a totally unexplored avenue in disintegrants. The widely used superdisintegrants are sodium starch glycolate, crospovidone LR (KOLLIDONE).
The reasons for the selection of Crospovidone are as follows: better compressibility compared with other superdisintegrants, high capillary activity, pronounced hydration capacity, and little tendency to form gels.
Moreover, the rate and extent of liquid uptake and swelling of Crospovidone LR (KOLLIDONE) are not reduced in 0.1 N hydrochloric acid when compared with aqueous medium. The aqueous medium (water) represents disintegration medium and 0.1 N HCl represents gastric environment.
Sodium Starch Glycolate is unimpaired by the presence of hydrophobic excipients such as lubricants (Figure 4).
Floating of cop+wax like material at different ratio result of preliminary trials for floating time study
In order to select the optimal ratio and process for Co-processed excipient preparation, of different ratios of Chitosan:Ozokerite wax, Chitosan:Carnuba wax, Chitosan:Compritol 888 ato, Chitosan:Cremophor RH 47 (Figures 5A-5D).
The best ratio of the co-processed excipients are Chitosan:Ozokerite wax (7:3).
Finally, co-processing is achieved by adding chitosan powder into the melted wax substance, with stirring, optionally followed by sieving.
It was found that the incorporation of hydrophobic water insoluble wax phase into a chitosan let to the formulation of co-processed excipients with good floating properties.
This allows the provision of a low density excipient matrix capable of making floating systems in the stomach.
Additionally, the chitosan dissolution which takes slowly place in the acidic medium of the stomach increases the porosity of the excipient matrix and further makes the excipient matrix less dense with time in the stomach.
Such an excipient matrix can be used to develop floating pharmaceutical compositions of drugs.
Physical properties
Bulk and tapped densities, compressibility index and the hausner ratio: Result of bulk, tapped, relative densities, carr index, and hausner ratio of the studied polymers and their Co-processing excipients are presented in table. The bulk, tapped and relative densities of the polymer increases as the co-processing excipients are increased.
Carr’s Index of ≤ 10 indicates excellent flow, whereas I values of 49.27-49.2 indicate good flow. Hausner Ratio of 1.1-1.125 indicates excellent flow whereas values of 1.28-1.375 indicate good flow.
The change in density before and after tapping calculated as % compressibility (Carr Index) is an indicator of how fast granules can flow to their highest packing. The carr index calculated from the density data showed a value less than 49.2 and hausner ratio of less than 1.1 further indicating the good flowability, which is an important factor for direct mixing powders. Good flowability of powder is needed for content uniformity and less weight variation in the final tablets . According to US Pharmacopeia 31, General Chapter <1174>, for Cop C:S powder are shown in Table 3 from the data obtained, Cop–C:S powder showed good flowability and compressibility [8].
Characterization of the compression process
Kawakita analysis: The fundamental knowledge on the compression and compaction of pharmaceutical powder is essential for the improvement and control of the final tablets and for the development of the compaction process. The compression behavior was analyzed using Kawakati analysis [9]. From the Kawakati equation and its linear form, according to equation:
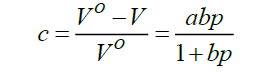
Where, V° is the initial volume and v is the volume of powder column under an applied pressure, P The parameter (a) represents the engineering strain (or degree of compression) at infinite pressure, while the inverted (b) parameter represents the applied pressure needed to achieve an engineering strain of a/2Equation (6) can be rearranged in linear form as:
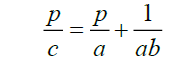
Parameter obtained from the linear Kawakita plots (r2 ≥ 0.9999) of the studied polymer and their Cop-C:S are presented in Table 2 With regard to constant a, Chitosan showed the highest compressibility (highest a value) followed by Syloid XDP 3150 [10]. When the co-processing Chitosan:Syloid XDP 3150 content was decrease from the different ratio 1:1,1:2,1:3,1:4 the a value , and therefore compressibility, of all investigated polymer underwent a decrease. The expression for particle rearrangement can be affected simultaneously by the two Kawakita parameter a and b. the combination of these into a single value, i.e. the product of the Kawakita parameter (ab) , may hence be used as an indicator of particle rearrangement during compression [11].
| Polymer/co-processing | Bulk Density (g/ml) | Tapped Density (g/ml) | Relative Density (g/ml) | Carr Index | HausnerRatio |
|---|---|---|---|---|---|
| Chitosan | 0.545 | 0.6 | 0.909 | 59.09 | 1.1 |
| Syloid XDP 3150 | 0.666 | 0.857 | 0.777 | 84.93 | 1.28 |
| Cop-C:S-1:1 | 0.4 | 0.5 | 0.8 | 49.2 | 1.25 |
| Cop-C:S- 1:2 | 0.5 | 0.666 | 0.75 | 65.91 | 1.33 |
| Cop-C:S- 1:3 | 0.363 | 0.5 | 0.727 | 49.27 | 1.37 |
| Cop-C:S- 1:4 | 0.444 | 0.5 | 0.888 | 49.11 | 1.12 |
Table 2. Cop–C:S powder showed good flowability and compressibility.
Table 3 shows the Kawakita Equation value for Chitosan, Syloid XDP 3150, Cop-C:S. The Kawakita constants a, b, ab and 1/b were calculated from the intercept and slope of the plots (Table 5). The Kawakita constant a, which represents the compressibility, is the height for Chitosan (a=4.966) and this is due to the large internal surface pores. The compressibility of Cop-C:S is higher than Syloid XDP 3150 (a=3.9875,2.966,3.9575,3.9725 and 3.4457) this result the fact that although the Chitosan constitutes (300 mg) and lactose are dilutes (200 mg) are mixed of Cop-C:S content, using Direct Mixing techniques in the preparation of Cop-C:S keep the large chitosan surface pores unoccupied and active [12]. The ab parameter suggest that the degree of particle rearrangement of all studied polymers decreased with increasing the Cop-C:S content. With increasing compression pressure, particle rearrangement becomes in significant and particle deformation and fragmentation becomes the dominating densification mechanisms .Particle rearrangement without parallel particle fragmentation or deformation represents one type of powder flow. The increase in the ab value for Chitosan (12.330578), Syloid XDP 3150 are the lowest volume reduction during compression with the lowest degree of rearrangement leading to a high densification powder packing followed by chitosan, Cop-C:S showed the highest volume reduction during compression. The 1/b parameter is an inverse measure of the amount of plastic deformation occurring during the compression process. Generally, a low value of 1/b is a reflection of the soft nature of the material and that the material is readily deformed plastically under pressure. The values of 1/b increased with increasing Cop-C:S content (Table 3)[13].
| Material | Slope | Intercept | a | b | 1/b | ab | r2 |
|---|---|---|---|---|---|---|---|
| Cop-C:S-1:1 | 0.2495 | 2.1027 | 3.987 | 1.993 | 0.501 | 7.95 | 0.972 |
| Cop-C:S-1:2 | 0.2506 | 1.4826 | 3.972 | 1.986 | 0.503 | 7.89 | 0.9999 |
| Cop-C:S-1:3 | 0.3367 | 1.3216 | 3.957 | 1.978 | 0.505 | 7.83 | 0.9993 |
| Cop-C:S-1:4 | 0.2522 | 0.4748 | 2.966 | 1.483 | 0.674 | 4.398 | 1 |
| Chitosan | 0.1863 | 11.646 | 4.966 | 2.483 | 0.402 | 12.33 | 0.9974 |
| Syloid XDP 3150 | 0.2672 | 18.521 | 3.445 | 1.722 | 0.58 | 5.936 | 0.9999 |
Table 3. Kawakita equation value for chitosan, syloid XDP 3150, Cop-C:S.
Preparation of powder for co-processed excipients of water penetration rate: Water penetration rate of Cop-C:S (different ratio) 1:1,1:2,1:3,1:4 and other polymer chitosan and syloid XDP 3150 was determined to further highlight the mechanistic action of co-processed excipients this is shown in Figure 6.
The choice of using Chitosan and Syloid XDP 3150 and Cop-C:S (different ratio) was based on its free allowance to water passage without any hindrance due to gelling.
This means that their presence within the limits cannot hinder the passage of water. It is only when their co-processed excipients are increased above their, water cannot longer pass through due to the swelling action of Chitosan, Syloid XDP 3150. However, this was only increased when Cop-C:S-1:4 higher than that, there would be a decrease in the rate due to Syloid XDP 3150. Cop-C:S-1:4 showed the highest water penetration rate without limits to its content (Figure 6) [14].
Testing the super disintegration power of cop-C:S-1:4 among commercial super disintegrats
Finally, the super disintegration competence of the naturally occurring the low concentration of chitosan-Syloid XDP 3150 (Cop-C:S-1:4) in tablets was as efficient as crospovidone and sodium starch glycolate. The most interesting feature could be seeing when disintegration time became elevated when the concentrations of the commercial super disintegrates were increased above their limits [15]. They are only unique and distinctive super disintegrate that maintained its functionality at a concentration ranges within the tablet dosage forms. Disintegration Time at different disintegration percentages is shown in Table 4.
| Material | 1% | 2% | 3% | 5% |
|---|---|---|---|---|
| Crosprovidone | 16.49 S | 20.12 S | 29 S | 32 S |
| Sodium Starch Glycolate | 10 S | 11.23 S | 15.20 S | 25 S |
| Cop C:S-1:4 | 30 S | 20 S | 10 S | 10 S |
| Placebo as blank | 10 S |
Table 4. Kawakita equation value for chitosan, syloid XDP 3150, Cop-C:S.
%Hydration capacity: The percent hydration capacity of the polymer and their co-processed excipients was evaluated. Result is presented in Figure 7. Syloid XDP 3150, decreased percent hydration capacities upon increasing the co-processed ratio 1:1 and 1:2. Such behaviour is mainly attributed to the increase in their swelling ability as previously illustrated. However, at co-processed excipients greater than 1:4, the hydration capacities of Syloid XDP 3150 and decreased. Chitosan are a different behaviour whereby the percent hydration capacity slightly decreased with increasing the excipients of co-processed ratio of 1:4 followed by a remarkable reduction at high co-processed excipients (Figure 7) [16].
Fourier-transform infrared study
Initially, the infrared spectra of Chitosan, Cop-C:S-1:4, Cop-C:S-1:1 physical mixture and Syloid XDP 3150 were performed and show in Figure 8.
The appearance of crystalline characteristics on Co-processed was further illustrated using FTIR spectrum analysis of the pure components and the co-processed (see Figure 8). Pure chitosan showed a distinct C=O and NH2 bands at 3821.54, 3743.68, 3616.08, 2795.99, 2353.24, 1694.69, 1527.79, 1390.32, 902.22, 656.89 respectively cm-1 (Cerai, Guerra and Tricoli, 1996). These bands were noticed to become sharper in shape on co-processed of Syloid XDP 3150 on chitosan, indicating the formation of crystalline compounds. In addition, the FTIR spectrum of Chitosan, Syloid XDP 3150, and Cop-C:S-1:4 co-processed did not represent a chemical reaction type.
The chitosan could be supported by the fact that there was reduction of the main amide peak at 1390.32 cm1 (Figure 8), as this band is normally known to be highly interactive [17].
Therefore, the FTIR of Cop-C:S-1:4 Co-processed was almost similar to that obtained by physically mixing the two components as illustrated in Figure 8. Hence, the preparation method did not involve any chemical reaction, and just a physical modification occurred on the particles’ surfaces. This close association between Chitosan and Syloid XDP 3150 and silica without evidence of covalent bonding (as indicated by the FTIR analysis) and ionic interactions (as Co-processed was brought to neutrality) suggests that silica or silicate ions interact with the glucopyranose rings of chitosan, presumably through dipole–dipole and hydrogen-bonding interactions. From the FTIR spectra shown in Figure 8, Cop-C:S-1:1 ratio physical mixture superimposition of the vibrational band profiles contributed by Chitosan and Syloid XDP 3150 (Figures 8 and 9) [18].
X-Ray diffraction
X-ray powder diffraction patterns composed of broad maxima. Typically, Chitosan showed six broad peaks at 11.2°, 15.2°, 21.6°, 25.6°,27.7°, 18.7°, which are likely caused by polycrystalline domains or disturbed crystal structure. On the other hand, Syloid XDP 3150 showed a broad band at 22.3°. It appears from the figure that the co-processed gained noticeable amorphorus characteristics as evidenced by the appearance of three sharp and narrow peaks at 29.5°, 32.3°, and 35.8°. This suggests that the acidic medium could contribute to the reduction of the powder’s amorphous region and the appearance of such crystalline characteristics, as the degree of depolymerization of the glycosidic linkage (present in chitosan) is generally enhanced by the acidic environment. The absence of new bands or shifts in the patterns indicates the absence of formation of a new crystal form or chemical interaction. This property is essential for such co-processed excipients to work as excipients (Figures 10A-10C) [19].
An X-ray Diffraction Analysis (XRD) is commonly used to study the structural properties of grapheme nanostructures. Figure 11 shows the XRD patterns for wax compounds, in which the characteristic peaks for these materials are presented. Chitosan co-processed on Ozokerite Wax particles has established partial crystalline characteristics of the physically crystalline chitosan. In fact, the term non-crystalline applies to materials that produce X-ray powder diffraction patterns composed of broad maxima. This was seen in the case of pure Chitosan (Figure 11A) and Ozokerite wax (Figure 11B) [20]. X-ray power diffraction patterns composed of broad maxima. Typically, Ozokerite wax showed six broad peaks at 35°, 65°, 101°, 137°, 174°, 210°, which are likely caused by long alky chain are crystal structure. Upon Co-processed, Chitosan-Ozokerite wax (Figure 11C) powder showed some degree of crystallinity attributed first to the sharp narrow diffraction peaks at 210° and 375° and second to the slight decrease in pure Chitosan crystalline peaks. Cop-C:O-7:3 due to the low content of Chitosan in the mixtures. The absence of new bands or shifts in the patterns indicates the absence of formation of a new crystal form or chemical interaction (Figure 11A-11C).
SEM (Scanning Electron Microscopy)
After drying and sieving, particle morphology of Chitosan, Syloid XDP 3150and Cop-C:S-1:4 Co-processed powder was analyzed using SEM technique and compared for particle size and shape with the original constituents. In (Figure 12 A) the thin flake-like granules with flat surfaces of Chitosan became more irregular with folded edges having three-dimensional appearance. Chitosan had changed its native structure from thin, flat surface structure to three-dimensional compacts. In (Figure 12 B) Syloid XDP 3150 content, the particles were more intense in white colour and many surfaces were dominantly flat. In (Figure 12 C) on the other hand, there was no appearance of the white Syloid particles in the same of Cop-C:S-1:4 particles, which were of regular surface and of various particle size, suggesting complete integration with or within the Chitosan particles (Figure 12A-12C) [21-24] .
In the present work we investigated co-processing Excipients were cop (C:S), chitosan and syloid XDP 3150, in weight ratios of 1:4 (w/w) and produced by direct mixing Differential Scanning Calorimetry (DSC), Fourier-Transform InfraRed (FT-IR), X-Ray Powder Diffraction (XRPD) and Scanning Electron Microscopy (SEM) techniques were used to characterize Cop-C:S and Cop-C:O, in addition to characterization of its powder and immediate release and controlled release dosage form.
[Crossref] [Google Scholar][Pubmed]
[Crossref]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar][Pubmed]
[Crossref] [Google Scholar][Pubmed]
[Pubmed]
[Crossref] [Google Scholar][Pubmed]
[Crossref] [Google Scholar][Pubmed]
[Crossref] [Google Scholar][Pubmed]