ISSN: 2347-7830
ISSN: 2347-7830
1Emeritus from Friedrich-Alexander University, Department of Biology, Neue Str. 9, 91096 Möhrendorf, Germany
2Department of Medicine and Pharmacy, University of Joinville Region – UNIVILLE, Rua Paulo Malschitzki, 10 Campus - Industrial Zone, PO Box 246, Joinville, SC CEP 89219-710, Brazil,
Received: 04/05/2015 Accepted: 11/06/2015 Published: 20/06/2015
Visit for more related articles at Research & Reviews: Journal of Ecology and Environmental Sciences
Worldwide rapidly increasing pollution from industrial, municipal and agricultural origin is a major threat to natural aquatic habitats, water reservoirs for recreational purposes and drinking water supplies. The multitude of organic and inorganic toxins cannot possibly be monitored by exhaustive chemical analysis. As a consequence a number of bioassays have been developed over the recent past to monitor aqueous ecosystems analyzing the biochemical and behavioral responses of organisms as early warning instruments to detect potential toxicants in the water. Photosynthetic motile flagellates (Euglena gracilis) have being used in the new generation Ecotox instrument by which 13 physiological and anatomical parameters are being evaluated as end points including motility, swimming velocity, gravitactic orientation as well as cell form and size. The instrument operates on line evaluating movement vectors of large numbers of organisms in video sequences in real time to warrant high statistical significance.
Aquatic ecosystems, Euglena, Ecotox, Flagellates, Bio monitoring, Ecotoxicology, Pollutants.
Even though two thirds of our planet is covered by water, only a small fraction of far less than 1 % is available as liquid fresh water [1]. However, the demand for fresh water for industry, households and agriculture have risen exponentially over the past 30 years and is expected to further increase [2]. The limited fresh water resources are further threatened by increasing pollution from domestic, industrial and agricultural wastes by heavy metals, persistent organic pollutants (POP), fertilizers, pesticides and pharmaceuticals [3]. The consequences of global climate change which affects rainfall patterns are predicted to worsen the situation [4]. Especially in developing countries the situation is dramatic since about 780 million people lack access to clean fresh water [5] and 2.2 billion lack safe sanitation [6]. Also these conditions are expected to further deteriorate due to global climate change [7]. Limited water resources, dropping ground water tables, rising temperatures and changes in precipitation are bound to decrease agricultural productivity [8,9]. WHO estimates that about 5 million people die every year from water-related diseases such as diarrhoea and infections by water-borne parasites .
Water pollution from toxic substances can result from a point source such as an effluent from industry [10]. Alternatively it can derive from spread sources such as agriculture or municipal wastes [11]. Zn, Cu, Pb, Cd, Hg, Ni, Cr and other heavy metals are widely occurring pollutants in aquatic ecosystems and drinking water reservoirs such as ground water, lakes and rivers. These substances enter the water from untreated effluents from industry or mining wastes [12,13]. Heavy metals tend to be bio accumulated in aquatic organisms and reach high levels in the food web such as fish or birds and pose a threat for human consumers [14]. E.g., Cd has been observed to accumulate in aquatic organisms with concentration factors ranging from 102 to 105 [15]. Similar concentrations of heavy metals were found in rivers in Pakistan and India [16,17].
Both persistent organic pollutants and inorganic chemicals are serious toxins in aquatic habitats as they can be enriched in sediments [18]. Alternatively they are concentrated in the food web and therefore present a threat for the whole biota [19]. Recently, personal care products and pharmaceuticals such as estrogens have become concentrated in river water where they are recycled and enhanced in concentration [20]. Hormones can reach concentrations where they induce feminization in fish and amphibians and have negative effects on humans [21,22]. Chlorophenol compounds are derived from industrial waste, pesticides and degradation products of chlorinated hydrocarbons [23]. They are considered as some of the most toxic pollutants because of their high toxicity, chemical stability and low degradability [24,25].
Because auf the increasing pollution of aquatic ecosystems monitoring and assessing drinking water supplies as well as waste water disposal have a high priority. Chemical analyses are expensive, time consuming and often fail to detect toxins because of the large number of potentially toxic materials [26]; in addtition, they may underestimate the toxicity of mixtures of chemicals which could act synergistically [27]. In addition, upper limits for pollutants in aquatic ecosystems vary between countries and over time and may not reflect the potential threat for the biota [28].
The alternative to chemical analysis is to deploy bioassys to monitor water quality. Many different types of bioassays have been developed using mortality, motility and behavior, growth and physiolocial parameters such as photosynthesis, protein biosynthsis and genetic alteration of aquatic organisms as end points [29]. Organisms used for this purpose include bacteria and microorganisms, insects, fish and mollusks as well as algae and higher plants which are employed to monitor toxicity of fresh water reservoirs for drinking or recreational use as well as to determine potentially toxic substances in municipal, industrial and agricultural effluents [30-32].
By definition, bioassays do not identify the chemical nature of a toxin in the water, but signal the presence of a pollutant and indicate the concentration range for adverse effects, e.g. by determining effect-concentration curves (EC curves). For this purpose a dilution series is performed which indicates the highest concentration of the pollutant which does not cause a detectable effect on the endpoint (no observed effect concentration, NOEC). Often the EC50 value is determined indicating the concentration which induces a 50% inhibition [33]. The concentration in a dilution series which causes 100% inhibition is called lethal dose (LD) [10,34].
One successful approach using photosynthetic flagellates was realized in the Ecotox system which monitors several movement and orientation parameters affected by a large range of potential pollutants using online real-time image analysis of the swimming cells [35,36]. The aim of the current work is to describe the newly developed new generation Ecotox system based on enhanced video technology and a novel image analysis system.
In principle, any type of motile organism ranging from bacteria to large animals could be used for this bioassay. Depending on the size of the organisms only the optical parameters such as magnification need to be adjusted. For example, the microcrustacean Daphnia is internationally regarded as a suitable motile organisms which is sensitive to many pollutants and toxins [37,38]. But also oganisms as small as motile bacteria have been used; in that case dark field illumination is preferable
The optical conditions described below have been optimized for the employment of the unicellular photosynthetic flagellate Euglena gracilis wild type strain Z. The culture was obtained from SAG (Sammlung von Algenkulturen at the University of Göttingen) [39] and grown in a medium published by Starr [40] at 22ºC under low light conditions (25 W m -2 from mixed warm tone and daylight fluorescence tubes) in static Erlenmeyer cultures. Cells used for experiments were >10 d old since they need some time after inoculation to develop a persistent gravitaxis (see below). Growth of the microorganisms is very simple and the cells grow rapidly, so that no complicated culture conditions need to be kept
The hardware of the new generation ECOTOX consists of an optical unit which connects an observation chamber, in which the flagellates swim, with a monochromatic USB camera (Point Grey, Blackfly BFLY-U3-13S2M-CS) with 1.3 mega pixel resolution (1288 x 964). A 10x microscope objective is used to project the image of the moving cells onto the CCD target of the camera (Figure 1).
The light source for monitoring is an infrared LED in order not to induce photosynthetic or phototactic responses of the flagellates, and the radiation is diffused by two Teflon disks. A stepper motor pump transports a fresh flagellate suspension into the observation chamber. A second pump is used to rinse the chamber and the connecting tubes, and a third transports the potentially toxic sample. Flagellate suspension, rinsing or diluting water and toxic sample are mixed thoroughly in a mixing chamber before entering the observation chamber. All three pumps are controlled by an Arduino Mega 2560 microcomputer based on the Atmel AVR microcontroller ATmega2560 which features numerous input/output (I/O) pins as well as a number of analog-to-digital (A/D) inputs. The microprocessor controls an electronic board (quadstep by Sparkfun.com) which can drive up to four stepper motors at 1.8° resolution (NEMA 17 DamenCNC, Alphen aan den Rijn, The Netherlands) with an attached peristaltic pump (PP60). The stepper motors allow precise dosing of the liquids in order to warrant exact dilution of the toxins. A 4 x 20 character LCD display indicates the current action such as which pump is active or which part of the program is currently running. The hardware plus a 3 A, 12 V switching power supply are integrated as a compact block which is housed in a container which also holds the containers for the fluids and the waste.
The software is based on the open source software Fiji which is a further development of ImageJ [41]. Normally ImageJ processes only recorded video .avi sequences; therefore a software plugin was used to allow direct input of the online video into the imaging software (Phase, Lübeck, Germany).
The ECOTOX software, written in the Macro Language of ImageJ, first starts the camera control and then allows the user to input a selectable number of frames (stack). After contrast enhancement and background subtraction each frame is thresholded resulting in white objects in front of a black background. From these frames all movement vectors of motile objects (up to 256) are analyzed. The angular direction of movement is determined.
Since the optical instrument is oriented horizontally and thus the observation chamber vertically, gravitactic orientation of the cells can be extracted. The Euglena cells can detect the direction of the gravitational field of the Earth and orient themselves accordingly upward or downward by an active process called gravitaxis [42,43]. The analysis is repeated cyclically until a predefined number of tracks have been determined or for a predefined length of time, whatever comes first. The direction distribution is displayed online in a circular histogram; the number of sectors can be determined by the user and updated after each cycle through a new sequence of video frames (Figure 2).
The direction of movement is calculated as the angular deviation α from the vertical.
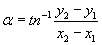
Where y2 and y1 are the end and beginning coordinates of each track, respectively, and likewise for the x coordinates [44]. The precision of orientation is calculated as the r-value, which is a statistical measure which runs between 0 (random orientation) and 1 (all cells move in the same direction).
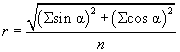
The mean angle of movement is given as θ.
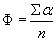
From the angles of movement the percentage of upward swimming cells (± around the vertical upward) is calculated. The swimming velocity is determined along the (meandering) total swimming path and as a direct line from the beginning to the end of the vector. The directedness is calculated from the ratio of the direct path length divided by the actually covered path. Filters can be defined by the user to limit the analysis to object larger than a minimal area and smaller than a maximal area in order to avoid tracking potentially disturbing particles such as bacteria or air bubbles. Also the swimming velocity can be selected to be in a defined window in order to avoid tracking e.g. non-motile, but sedimenting cells. The motility is calculated as the percentage of moving organisms (within the size definition) from all objects detected.
The area of the all analyzed objects is calculated. In addition, the perimeter (length of outside boundary), the circularity, roundness, solidity and aspect ratio (ratio of the major and minor axes of a particle’s fitted ellipse) are calculated as well as the Feret’s diameter which indicates the largest dimension of an object and the Feret angle, which is the angle of the longest axis with respect to the horizontal.
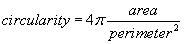
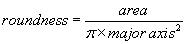
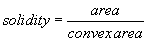
For all determined parameters mean values and standard deviation are calculated. Due to a size calibration the movement and size parameters are calculated in actual physical units (μm, μm2, μm/s)). The final result sheet is saved on hard disk with the time and date of the experiment as well as a comment of the user on the experimental conditions.
The ECOTOX system can run in several modes. Either a single experiment is carried out on cells in a given situation. Alternatively, the system can be programmed to first pump an unpolluted cell suspension (control) into the observation chamber where it is analyzed. After this a fresh cell suspension is mixed automatically with a polluted water sample or toxic substance and then analyzed. And finally a dilution sequence can be performed where the concentration of the toxin increases according to a predetermined sequence.
These pump functions are performed by sending a command via USB from the main program in the PC to the Arduino microprocessor, which echoes the command to ensure that it was received correctly. First of all, the serial port to which the microprocessor is connected is determined automatically. The commands for the motor control include, among other details, the selection of the motor (for cell suspension, rinsing and dilution water and toxic sample), run time for each motor (which equals the volume of liquid pumped) and the direction of pumping. Furthermore the temperature of the culture in the observation chamber can be requested. This is displayed on the LCD display of the ECOTOX hardware as well as sent to the main PC program which stores the value in the results file.
The data of the ECOTOX system can be used to calculate effect-concentration curves (Figure 3) and obtain information on toxicity like NOEC, LD and EC50 values [36]. The system can be applied for short-term as well as long-term (online) monitoring of pollution in surface or wastewater.
As compared to other commonly used bioassays, the main advantages of ECOTOX are low costs and short measurement times [10]. In contrast to some other systems, the operational costs are almost negligible and involve only keeping a Euglena culture. Since the image analysis and movement parameter calculation are performed online by real time image analysis, a complete measurement cycle including control and sample, takes about 6 to 10 minutes [35,45]. Running the system is user friendly and does not require extensive training of personnel. Due to the fact that the system performs all analyses fully automatically, user measurement and interpretation errors are avoided. Using a full range of 13 movement and orientation parameters as end points enhances the reliability and sensitivity of the ECOTOX system [10]. The sensitivity of ECOTOX to wastewater samples was found to be higher than other bioassays using such as algae (growth), Daphnia (motility), fish (mortality) and bacteria (bioluminescence) [36]. By definition, a bio monitoring system does not identify the chemical nature of a pollutant, so that a chemical analysis is required if and when a potential threat is detected. But nevertheless, since different motility and orientation parameters show different sensitivities to various groups of toxins, a first indication of the potential group of pollutant can be deduced.
The first generation of ECOTOX has been utilized to detect the biological consequences of a wide range of pollutants such as heavy metals, herbicides, pesticides, fertilizers, detergents, wastewaters and many other organic and inorganic pollutants [10,46-48]. It has been effectively used to test both short- and long-term ecotoxicity of pollutants [10,49]. The bioassay can be utilized as an early warning system for pollution detection in surface, municipal and industrial wastewaters as well as for monitoring efficiency of wastewater treatments plants. Because of its low cost, the system can be employed also in developing countries where poor funding and lack of environmental experts hamper an efficient water quality monitoring.
In the current configuration the ECOTOX system uses Euglena as a model organism, but it can be easily adapted to other small motile organisms by changing the dimensions of the observation chamber and camera magnification. The ECOTOX instrument and software can be obtained in various configurations from Real Time Computer (Möhrendorf, Germany, www.dphaeder.de).